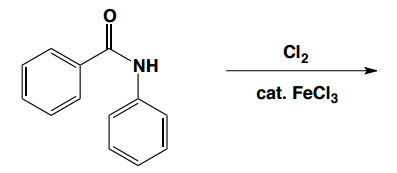
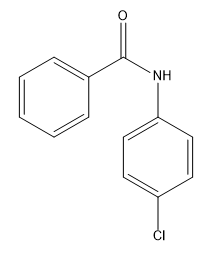
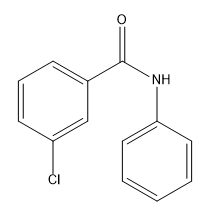
Now we know how to add a single substituent to a benzene ring using an EAS mechanism. We're pretty good at figuring out how to add different types. You know how to add nitros and R groups and ketones. But now the question we have to ask ourselves is, what happens if you want to do a second reaction on that benzene? What if you already have something there and now you're adding a second EAS reagent? Where is it going to add? How is it going to add? How is that first substituent going to affect the second? It turns out that it really has a huge effect on the second one and that brings us to the EAS of monosubstituted benzene. Now we're not talking about just regular benzene. We're talking about benzene with a substituent already. This section also is called directing groups or activity of benzene. All of that is covered in this section. It turns out that that first substituent is going to really alter the electron density of the benzene ring. So it's going to affect the reactivity towards subsequent reactions and it's going to affect the direction of subsequent reactions.
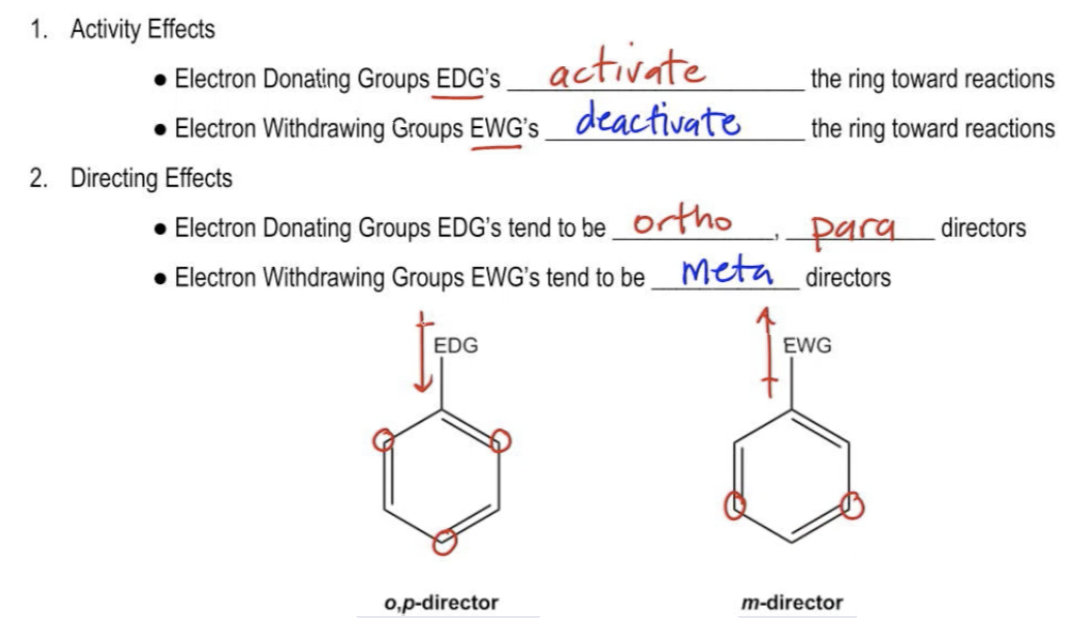
The first thing that it's going to do is it's going to affect activity. It turns out that this is our first introduction to these terms of electron donating groups or electron withdrawing groups. These are extremely important principles in organic chemistry and they're going to come up not only for the rest of this course but also for the rest of your professional career. If you want to go into anything pre-health and you need to take more classes in the sciences and in the life sciences, then you're going to need to know about electron donating groups and electron withdrawing groups.
First of all, if that first substituent that you add happens to be an electron donating group, that means it's giving more electrons to the benzene. Do you think it's going to make it more reactive or less reactive towards another EAS reaction? Remember, the benzene acts as a nucleophile in the reaction. The more electrons you pump into it, the more you're going to activate it to react. They activate the ring towards more reactions. So if you add an electron donating group, it's going to want to react even more the second time.
However, if you add an electron withdrawing group, that's going to pull electron density out of the ring, making it less nucleophilic. That's going to deactivate the ring towards future reactions. That means the second reaction will be more difficult to perform than the first, meaning it's actually less reactive than benzene by itself.
But that's not it, guys. You might be wondering, how do I know if something is electronegative? We'll get there. Just hold on. Also, guys, they have directing effects because it turns out that electron donating groups tend to be what we call ortho para directors. They tend to direct towards the ortho and para positions, whereas electron withdrawing groups tend to be meta directors, meaning that they direct subsequent EAS reactions to happen only at the meta positions.
Here I have a picture of these 2 benzenes and an electron donating group, we would expect to add the second EAS reagent in the ortho positions or in the para positions. Hence, OP director. It actually means that it directs to all of those positions. Whereas meta directors, electron withdrawing groups, they pull electrons out of the ring, so they're going to tend to add in the meta positions.
I forgot to draw the dipole of the electron donating. It's going to push electrons into the ring. It directs towards OP whereas electron withdrawing groups direct towards the meta positions. The scientific explanation of why that happens is going to be for another video. We're not going to really talk about the scientific technical definition right now. It has to do with resonance structures. But you could also read your textbook if you want more information on that. What I'm going to focus on for right now is really just memorizing and really just knowing which groups are your electron donating and which groups are electron withdrawing groups. So let's move on to the chart that's going to help us with this information.