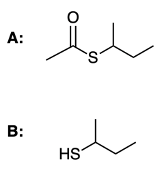
Hey everyone. So in this video, we're going to take a look at the hydrolysis of thioesters. Now, recall that thioesters are sulfur analogs of just regular esters. And we're going to say here that acid-catalyzed hydrolysis of a thioester produces a carboxylic acid and a thiol. And we're going to say here that the reaction takes place via a nucleophilic acyl substitution mechanism.
If we take a look at the basic premise of a NAS or nucleophilic acyl substitution mechanism, we have step 1 which is protonation, step 2 which is nucleophilic attack, step 3 is yet again a proton transfer, but here we're saying proton transfer because we've already done protonation in step 1. We're going to say step 4 is a leaving group, and then we're going to say step 5 is deprotonation. Steps 1, 3, and 5, although their names are different, they're basically talking about the movement of a proton, an H+ from one structure to another. Right? So they have that in common with each other even though the names are slightly different from one another.
The premise still remains. Right? So when we're talking about acid-catalyzed hydrolysis of thioesters, this is going to be the basic setup for a majority of the mechanisms that you're going to see.