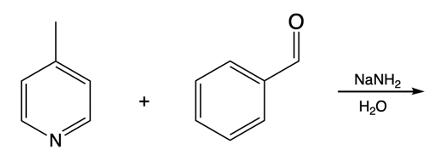
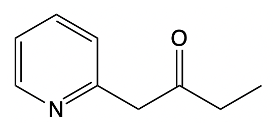
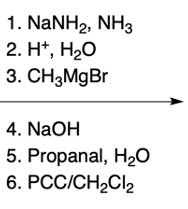
Hey, everyone. So in this video, we're going to talk about side chain reactions of our substituted pyridines. Now remember that benzylic hydrogens are acidic due to stable intermediate anions. And remember, the benzylic position is the carbon that's connected to an aromatic ring. In this case, we're not just dealing with benzene; we're dealing with pyridine because pyridine is also an aromatic ring.
Now we have our ordinary benzylic carbanion, so those are the ones that are attached to benzene. Well, they're stabilized by resonance, which is great, But pyridine carbanions are even more acidic because not only are they stabilized by resonance, but they're also stabilized by induction of the electronegative nitrogen atom. The creation of this negative charge when we remove an H+ can be stabilized by the more electronegative nitrogen. This is displayed in the pKa values of our ordinary benzylic carbanion versus our pyridine carbanions. So if we take a look here, in both of these, we have a base, and they will deprotonate the benzylic carbon in both of these structures.
So the base comes in, deprotonates, the Carbon holds on to the electrons. What we get here is our ordinary benzylic carbanion, and its relative pKa is around 41. For pyridine though, we deprotonate as well. We're going to form our own carbanion. It has a pKa of around 34 to 36 or so, and we'll see why later.
But we see, nevertheless, that it has a lower pKa than our typical benzylic carbanions. Remember, lower pKa means more acidic. We're going to say here that the benzylic position can be deprotonated by strong bases, and we can see that in the form of organolithiums and also the strong sodium amide base. Alright.
So just remember, we can make our different types of carbon ions from these benzylic positions. When it comes to pyridine, they're even more acidic because of resonance and the inductive effect of the electronegative nitrogen atom.