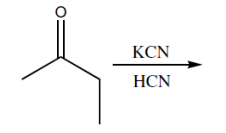
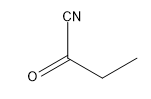
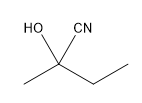
Now that we understand the general mechanism of nucleophilic addition, it's important to really understand those specific nucleophiles that can attack carbonyls and make substituted alcohols. One of the most famous of these is cyanide. Cyanide is a negatively charged nucleophile that's used to make a functional group called the cyanohydrin. You might already kind of be able to guess what that is just by the name but I'll show you. Typically, CN is reacted as a negatively charged anion. I mean NaCN is very common. We could also see KCN. This is a very common way to represent it as well. As you can imagine, this is just straight up nucleophilic addition. I've got my CN negative. I've got my very strong partial positive charge. I get a nucleophilic addition mechanism. What this is going to make is a negatively charged oxide and my CN substituent. That is what I get after the first step. Then there's always going to be a protonation step that you can use. You can use water or some kind of mild acid to protonate. Obviously, that's not the mechanism for protonation. It would be something like this and like that. You would get your functional group. Your functional group, whenever you have a CN and an OH on the same geminal to each other, that's called a cyanohydrin.
Now, I wanted to inform you that there's another form of CN that's actually pretty common as well that doesn't require a protonation step. That one, I'm going to erase a few arrows if you don't mind. You can keep the ones that you have on your page. But just want to show you guys another example would be if I used or if I used HCN. HCN is a really interesting compound because if you just look at it, you might think, "Oh, that's a source of CN negative. But remember that the carbon-hydrogen bond is actually a very strong bond. Usually, carbon and hydrogen don't just ionize like that. That's actually a covalent bond. Covalent bonds usually don't just dissociate to make H+ and CN-. That's kind of strange. Why would it do such a thing? The answer has to do with acidity, guys. It turns out that it's not that it's a weak bond in terms of polarity or that there's a dipole but it turns out that there's a very acidic bond. It turns out that HCN, if you guys remember your pKa's way back in the day, has a pKa of about 10. In normal aqueous environments, it's going to be ionized. It's going to be in an ionized form. Now the advantage of using HCN is that look what you got. You've got the CN- and you've also got your proton to protonate. You could do NaCN and then water or you could just do HCN. Gosh. That's not what I meant to do. Or you could just use HCN and HCN will take care of both steps. It will do the nucleophilic addition and you'll go ahead and you'll add your hydrogen for the protonation step.
Now what I want to do is, I specifically want to talk about 2 other reactions that happen with cyanohydrins that really have nothing to do with the nucleophilic addition. But as a functional group, we should be aware of what you can do to a cyanohydrin. That's what we're going to do in the next two videos. Let's go ahead and start off with the first cyanohydrin reaction.