Now that we understand nucleophilic addition to carbonyls, I want to focus on one of the most important nucleophiles that can react in nucleophilic addition: organometallics. I know organometallics can be intimidating. They look strange with a lot of atoms, including metals. However, these are some of the easiest compounds to work with in terms of pushing arrows because organometallics are really strong nucleophiles. We will do the same things with them as we would normally do with anything that has a negative charge. Organometallics participate in many different reactions. If you want to thoroughly review organometallics, you should go back to that section of your text or Clutch videos. I have plenty of videos on them. But what I'm going to focus on right now is just a refresher of what they do to ketones and aldehydes.
Remember that we can use the letters R and M to represent any organometallic because there's always going to be some kind of R component, some kind of alkyl group and some kind of metal. The two most common in this section are Grignards and organolithiums. A Grignard could be represented as R-Mg-Br. I'll write it down. Then there's organolithium, very straightforward. These molecules are extremely similar in how they react because they both have an ionic bond with an extremely strong dipole toward the R. In your periodic table, the further you go towards fluorine, the more electronegative elements become. Well, carbon is actually pretty close to fluorine, whereas these group 1 and 2 metals are some of the least electronegative atoms possible. This leads to a very ionic bond towards the carbon. So ionic, in fact, that we can write these things ionized. We can write it as R- Mg+Br. The same thing with organolithium, we write it as R- Li+. As such, these are just both a source of R-.
When that R- sees the carbonyl, we are going to carry out nucleophilic addition. We will attack the partial positive carbon, move the electrons up, and what we will get is a tetrahedral intermediate, O- R1 R2, with my new R group added. This tetrahedral intermediate would normally stay there but is usually followed by a protonation step. I don't know exactly what the protonation step will be but I'm going to put some kind of protonation would occur and you would get your substituted alcohol, O H R1 R2 and the R that we added. Just a reminder: you should have worked with organometallics before in this course. This is what you need to know for this section on how to add an organometallic to a ketone or an aldehyde. You will always get an alcohol product and a new R group attached to that tetrahedral carbon in the middle.
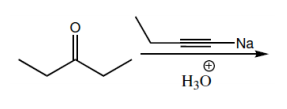
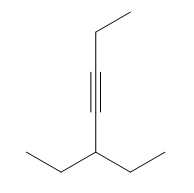
That being said, why don't you guys take a look at the molecule below, try to do the whole reaction, draw the mechanism, and then I'll show you the answer. Go for it.