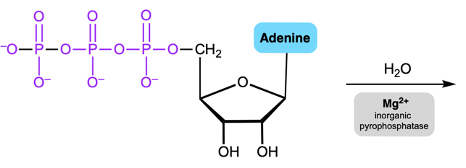
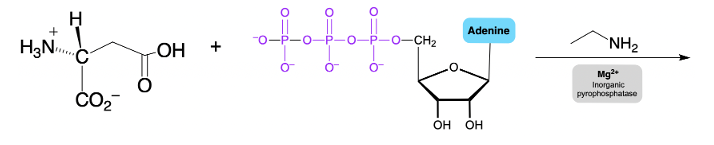
Hey everyone. So let's take a look at the chemical reactions of Phosphate Anhydrides. Now we're going to say here that the multiple negative charges of a Phosphate Anhydride make its phosphorus atoms resistant to nucleophilic attack. Now here we're going to say we have a magnesium ion and a class of enzymes called inorganic pyrophosphatases, it reduces the overall negative charges. Remember, a nucleophile has electrons.
It can be a neutral nucleophile, but usually the best nucleophiles are negatively charged. It's hard for a negative nucleophile to come in and attack a structure that's highly negative. Remember, like charges repel. So here we'd have to use magnesium ions and this class of enzymes to help reduce the negative charge of our phosphate anhydride. Here we'd say they help to create a complex which is our phosphate anhydride magnesium complex, the ionic bonds that form between our phosphoryl oxygen atoms and the magnesium ion.
If we take a look here, we have the magnesium ions forming ionic bonds with two phosphoryl oxygens furthest from the R group. If we take a look here, we have initially our alkylated phosphate anhydride and we can see that we have a bunch of negative charges. A nucleophile will not want to come in and try to target any of these phosphorus atoms like charges repel. So we're using this magnesium coupled with these classes of enzymes. Magnesium typically represents like a silver string.
If you've done the hydrochloric acid magnesium lab experiments, you might have used a little piece of magnesium strip. We see that it's silver in color which matches the silver color that we see here. It's kind of in a mesh with the enzymes themselves. But what effect does this have? Well, here, the magnesium ion comes in and it forms an ionic bond with these oxygens.
These oxygens, we realize that an ionic bond is not a solid bond, we have these dotted lines to represent that, But these two negative oxygens are kind of occupied at the moment with this magnesium. This lowers the amount of free negative charges on the surface of this phosphate anhydride, reducing the overall charge. Because there's less free negative charge, a nucleophile is more likely to come in and want to attack a phosphorus atom. So here, once we've added the magnesium and it forms these ionic connections with these oxygens, that's what we call the complex. So again, anytime we're dealing with a phosphate anhydride, we have to do this first.
We have to introduce the magnesium with this class of enzymes to help lower the overall negative charge before a nucleophile can come in and target any of these phosphorus atoms here. Alright. So just keep that in mind when we're dealing with these different types of chemical reactions.