Now we're going to learn how to name anhydrides. Anhydrides are a specific functional group that come up a lot in this section and they're one that you might not be very familiar with at all. Let's just back up for a second and talk about what an anhydride is. Basically, an anhydride is a carboxylic acid derivative. Think of it as a carboxylate. But then you've got an acyl group on the other side. Then you've got your carbonyl and R. Another way to think of it is that it's a dicarbonyl with an O in the middle. Whatever you want to think. In terms of naming, the naming of it is going to be visualized in this manner. Basically, when you look at an anhydride, it's actually kind of a combination of 2 carboxylic acids. You could say that maybe this was carboxylic acid 1 and maybe this was carboxylic acid 2. Maybe they came together and made an anhydride. Side note, that's actually how you make anhydrides. You make anhydrides by combining 2 carboxylic acids into an anhydride. That's where the name stems from. What we do is it's really easy. You just alphabetize your 2 different acids, visualize them with the carbon chains. But then instead of ending with the word acid, you would end with the word anhydride.
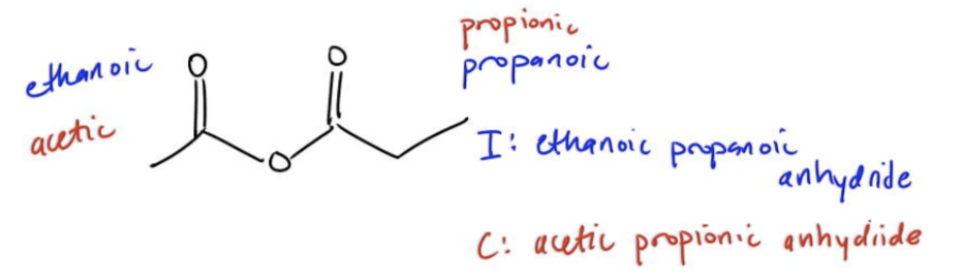
Another special situation is what happens if your R groups are the same? What happens if both sides of my anhydride are symmetrical? Well, then you don't have to say that it's carboxylic acid 1, carboxylic acid 2 and anhydride. You could just say carboxylic acid 1 anhydride. So just alkanoic anhydride. Why? Because that means that you're assuming that 1 combined with another version of itself to make an anhydride. You're basically saying that this is the anhydride you would yield through the condensation of these 2 carboxylic acids. Let me see. Obviously, just going really I don't want to mess up any questions for you. But this anhydride, I could name it as both the common and the IUPAC. Let's start off with IUPAC. For IUPAC, I would have this would be ethanoic here. And this is propanoic. For IUPAC, let me give myself a little bit more space. For IUPAC, it's going to be ethanoic, propanoic, anhydride, not acid. Don't make that mistake. Common. Common is going to be acetic and propionic. These are the ones you're supposed to memorize. Again, alphabetical order. We're going to get acetic, probionic anhydride. Now you see why it's so important to know those first five for the common names because they can come up with all of these derivatives. That's it. Move on to the question. Let's see if you can get it right and then I'll give you the answer.