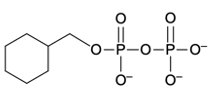
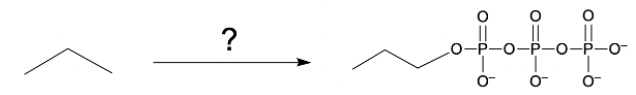
Hey everyone, so let's talk about a Phosphate Anhydride. Now, we're going to say a Phosphate Anhydride consists of 2 or more phosphate groups linked together. We're going to say the simplest neutral form is our pyrophosphoric acid, and it's formed by the condensation reaction between 2 phosphoric acid molecules. So if we come here and take a look, we have 2 phosphoric acid molecules. We're using H+ catalyst in order to initiate this condensation reaction.
Remember, in a condensation reaction, we're just essentially losing water. So let's say we lose water this way. What happens is we lose water so those 2 separate molecules come together, join together to create our one new molecule. Here, we'd still have our OH groups. This is its acidic form so the OH groups, they're protonated.
This would represent our pyrophosphoric acid. And here, this would be a phosphate anhydride. Remember, an anhydride, we learned about it being a carbonyl connected to oxygen connected to another carbonyl. So this is a typical anhydride. We're dealing with a phosphate anhydride now.
So we have phosphorus coming in and being substituted in. Now, here we're going to say the slightly basic environment of biological systems makes the pyrophosphate predominant. So here instead of having our OH's protonated, they're deprotonated because our biological systems are slightly basic around 7.4, so we'd have all negative oxygens. This would be our pyrophosphate. Now, we've actually been dealing with our phosphate anhydrase for quite a while if you've taken biology and other science courses.
We know about ATP, which is Adenosine Triphosphate. It has a portion of it which is a phosphate anhydride. It was there the whole time. Now, here we would say with our ATP which is Adenosine Triphosphate, so ATP, We'd have these negative oxygens because again, the basic environment that they exist in. And then we'd have our 5-membered ring.
We'd have it as a ribosugar. And then we have our nitrogenous base here in terms of adenine or adenine. Right? So again, we know what a phosphate anhydride is now, how it's created, and remember, ATP is the most important, most common type of phosphate anhydride. Followed by ADP, which is Adenosine diphosphate where we've lost one of these phosphate groups and now we only have 2.
Right? So ATP is the most important followed by ADP. And so keep that in mind when we continue our exploration and discussion of phosphate anhydrides.