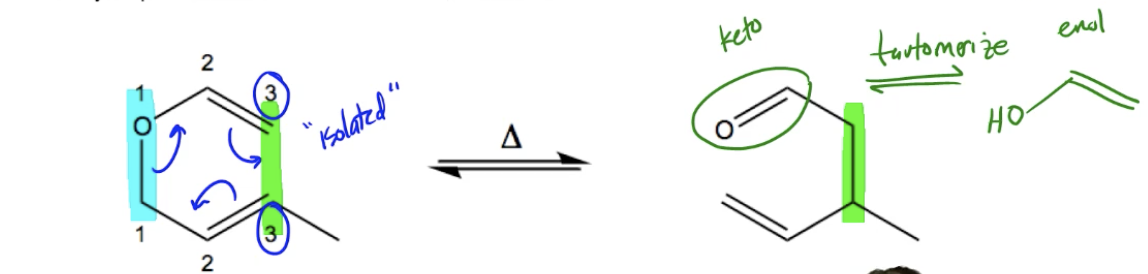
Hey everyone. In this video, we're going to discuss a specific type of sigmatropic shift called a Claisen rearrangement. So guys, what is the Claisen rearrangement? Well, it's a heat-activated, heat-activated, 3,3 sigmatropic shift that involves an allyl ether. So this is interesting. It's a specific type of sigmatropic shift. It's a 3,3, but specifically, the reactant needs to have an allyl ether in the original functional group to then transform into a Claisen rearrangement. If you don't have that allyl ether, you're sunk. It's not going to be a Claisen rearrangement. Okay? I will go ahead and help you identify the allyl ether in a second, but let me just read through a few more points first about it.
So depending on how many pericyclic reactions you've had to learn at this point, you might be kind of confused about how to identify which type is which. Well, something that's easy about the Claisen is that it's one of the few pericyclic reactions that does not begin with any form of conjugation. So, notice that we have a diene in here, right, but this diene is considered to be isolated because they're not next to each other, right? So it's really the only pericyclic reaction that could possibly take place between an isolated diene with an allyl ether. If you think about it with those two things in mind, you can easily determine this is going to be a Claisen rearrangement.
Now, something else to keep in mind is that right now I've drawn this molecule very nicely, so it's very easy to kind of think mechanistically how it would form into this product because the double bonds are close to each other. But just so you know, the way your professor or your homework may give you the problem, it may require some sigma bond rotation for you to get it to look nice and orderly before you do your mechanism. So I'll show you a few examples later about how we're going to have to rotate it first into position before we can then draw the mechanism. Okay. Cool.
So let's go ahead and just identify first of all, what do I mean by allyl ether? So guys, an allyl ether is, like I mean, let's just go all the way back to the beginning. What's an ether? An ether is an R-O-R group, an ether. And specifically an allyl ether would mean that your O is attached to a CH2 that's then attached to a double bond. Okay, so that's what an allyl group is. This group right here would be allyl. You should be familiar with allyl positions by now because we use them a lot in organic chemistry. But it means you're not directly attached to the double bond, you're one carbon away. So what is the allyl ether in the original reactant? It's this thing. The other thing on the top is actually part of a vinyl ether and that part actually could change. There are Claisen rearrangements that happen with vinyl ethers, there are Claisen rearrangements that happen with phenyl ethers, so don't worry too much about the top part because that part could change based on the specific type of Claisen. But the bottom part, that allyl part, always has to be there. It always needs an allyl ether, so that's why that's like the one defining characteristic of a Claisen rearrangement.
Now once again guys, you guys should already be familiar with how to number sigmatropic shifts in general, but remember that it has to do with the bond that is being broken and the bond that is being made. So in this case, the bond that is obviously broken is the one between the ones because you can see over here it no longer exists. The bond that's being formed is the one between the threes because now that's a new bond over here. So we would then say that this is in the category of a 3,3 sigmatropic shift because a new bond has been created between the threes. Cool. And lastly, what would this mechanism look like? In general terms, it would just be any mechanism you can draw that's pericyclic and concerted that is going to create a bond and break a bond. So what I would do is something like this, something like this, and something like that. Cool? And there you have it, that is your final product.
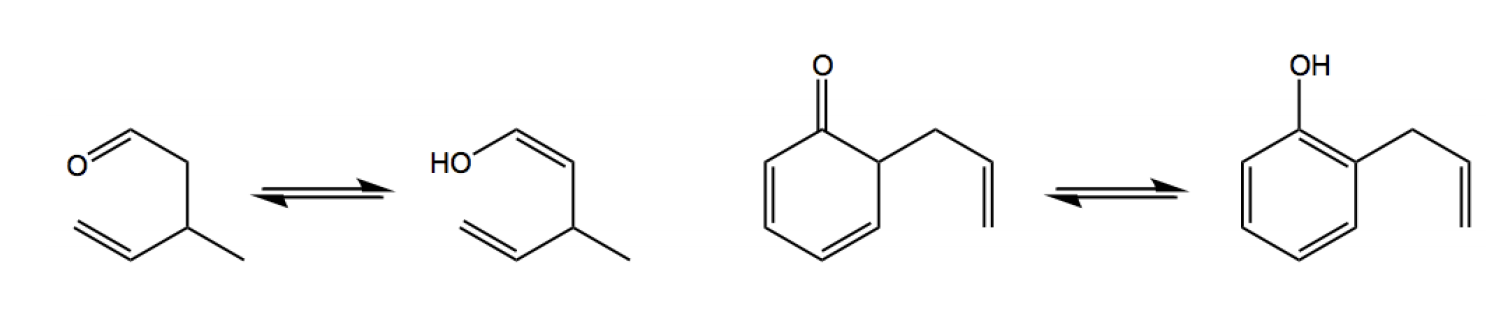
Now guys, it turns out that it can get a little bit more complicated than this though, and that's because sometimes in your final product, you're going to end up having to decide which tautomer is the most favored because if you'll notice, we actually have a carbonyl, we have a carbonyl as our end product here. Okay. We have a carbonyl and something that we learned about carbonyls a long time ago, or I mean you've definitely come across it at some point in organic chemistry, is that carbonyls are able to tautomerize into enols. So they're able to tautomerize into enols. And this is, so this is called the keto and this is called the enol. Now, most of the time, the keto is favored, almost all the time the keto is favored. So you usually don't need to worry about it. Usually, you can just leave the product the way it is because ketos are favored, but some specific molecules prefer the enol. So what that means is that when you come across a molecule that favors the enol position, you must tautomerize to the enol in the last step. If you don't tautomerize to the enol, you get the question wrong because you picked the wrong tautomer. Okay? So what it says here is that in the final tautomerization, a final tautomerization step is required for molecules in which the enol form is favored. Again, not too many molecules favor the enol form, but if they do, you must tautomerize to the enol. Makes sense? Cool. So what we're going to do in the next practice problem, in the next example, is I'm going to remind you guys of some of the properties of keto-enol tautomerism and we're going to pick the most favored one at equilibrium.