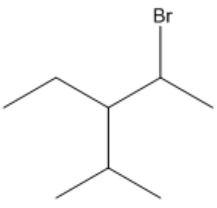
So I've got some good news. We're all done with alkanes. The bad news is that now we have to add some functional groups to the mix. Alright? So the first and easiest one we're going to start off with is alkyl halides. Alright? So alkyl halides are simply named by adding them as a substituent before the root chain indicating their location. Okay? So what that means is that remember that at the beginning of this lesson we talked about substituents and modifiers? Alkyl halides don't have modifiers. They're just called substituents just like an alkane would be, like an alkyl or whatever. Okay?
So one thing we should know is our prefixes for these different halogens. So fluorine would get the prefix fluoro. Okay. Chlorine gets the prefix chloro. Bromine gets the prefix bromo. And then iodine gets the prefix iodo. Okay. And these are going to be the prefixes that we use to assign the name to the alkyl halide. Okay.
The next thing that you should know is that alkyl halides have no priority. Zero priority when it comes to numbering the direction of the chain. So what that means is a lot of people think oh, I've got this long chain and this side has a chlorine on it, so that must be the side that I start from. No. Not at all. Use the same rules that we did before. Just you look at the closest substituent. It doesn't matter what it is. It could be a chlorine, it could be a methyl, it doesn't matter. You still give your lowest your you still make the chain start from the side that has the closest substituent.
So what I want to do is apply this to this alkyl halide. Go ahead and try to solve it out on your own first, draw it out and then when you're done, you have your answer figured out, then go ahead and go to the next video.