Everyone. So in this video, we're going to talk about the directing effects within our common heterocyclic compounds. Here, we're going to pay close attention to our ortho, meta, and para positions in these 5-membered heterocycles. Now ortho, meta and para terms are limited to substituted benzene rings. However, identical numerical relationships exist for 5-membered aromatic heterocycles.
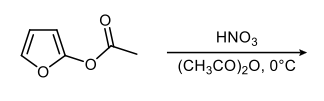
Here, we're going to compare our substituted benzenes to these substituted heterocycles. For the first one, we have these 2 methyl groups, and we're going to say they're ortho to one another, which means they are C1C2 to one another. We can make this one 1 and this one 2. Now, this is important. When it comes to substituents' relative positions, they're assigned through the carbon skeleton not through the heteroatom.
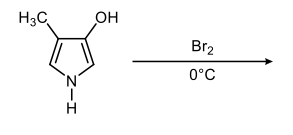
Now, remember when we're naming these heterocyclic compounds, we make the Heteroatom location number 1. But we're not trying to name these heterocycles, we're just trying to talk about directing effects. So just as in Benzene, these methyl groups are ortho to each other, they're also ortho to each other on this heterocycle. We're going to make this one 1 and this one 2. In the next one, we have carboxylic acid and a nitrile group.
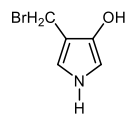
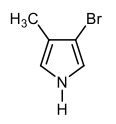
They are meta to each other, C1C3. Just like I'm going to say these 2 are meta to each other, C1C3. And then finally, these 2 bromines are C1C4 to each other which is para. And we're going to say these are C1C2C3C4, also para to each other. Again, this is how we're going to look at the numerical relationship between our substituents on these heterocyclic compounds because that'll help us to apply directing effects to the heteroatom as well as the substituents later on.
Alright. So again, this has nothing to do with the naming of these heterocycles. This is just helping us to apply them to directing effects.