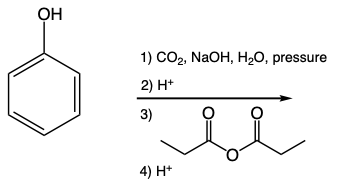
Hey, everyone. In this video, we're going to talk about the Kolbe-Schmitt reaction. Now EAS or electrophilic aromatic substitution reaction is used to synthesize ortho-hydroxybenzoic acid, otherwise known as salicylic acid. We're going to say under basic conditions, phenol will be deprotonated to form phenoxide. Now, phenoxide is then carboxylated, electrophile at the ortho position.
Now, here we have our phenol. Here we have carbon dioxide. We're utilizing sodium hydroxide with water and a proton followed by high pressure. This will help us to force the carbon dioxide onto the benzene ring and then having it be protonated into a carboxylic acid at the end. That's essentially the Kolbe-Schmitt reaction.
One key thing to take away from this is we have a phenyl group which is an ortho-para director. Ideally, when we want to add a group to benzene, it would be most advantageous to put it here in the para position so that we can avoid steric clashing. But with the Kolbe-Schmitt reaction, we actually make an ortho product. The reason for this is because of intramolecular hydrogen bonding. So the oxygen of this carbonyl could form an H-bond with the H of the hydroxyl group.
This is intramolecular, meaning within the same molecule. This actually leads to greater stability of the overall molecule. So we have a case where the ortho position is actually better for these two substituents. So, again, remember, OH is an ortho-para director. Typically, we want the next group to go in the para position, but if there's intramolecular hydrogen bonding, that's possible.
It'd rather go ortho. The Kolbe-Schmitt reaction takes advantage of this idea. Right? So just keep that in mind. The whole point is to go from phenol to this salicylic acid.