Remember that Lewis structures were ways to describe molecules where everything was drawn explicitly. That means that all bonds, all lone pairs, and all atoms were clearly shown on the structure. Back in the day, you learned a set of rules in general chemistry that you're supposed to remember about computing the theoretical number of valence electrons and then subtracting electrons and stuff like that. But if you already have knowledge of the octet rule and bonding preferences, it turns out that we can do almost all of these Lewis structures just based on that knowledge. So I'm going to go over these rules just to remind you, but I'm also going to show you that now, in organic chemistry, we have an easier method to do this based on the new information you've learned about bonding preferences.
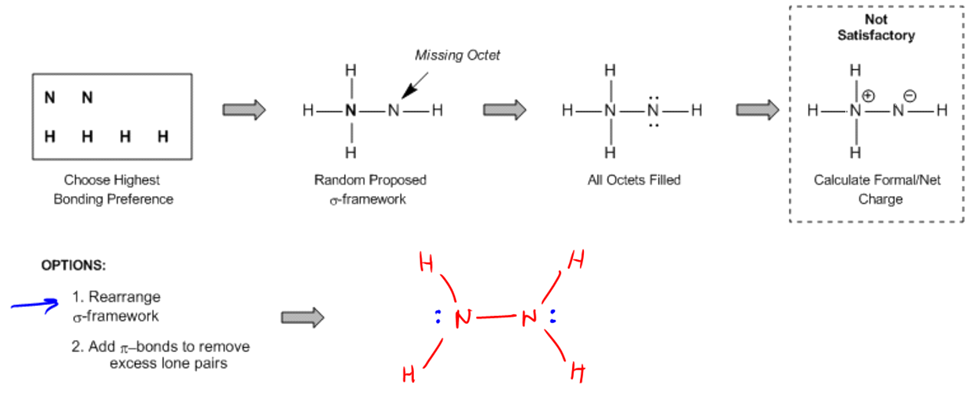
Remember, the first thing you always had to do was if you're given a molecular formula and you're trying to convert that into a Lewis structure and trying to figure out what the Lewis structure looks like, you always start with the atom that has the highest bonding preference in the middle. In your class, you may have learned that it is either the most electropositive atom or the one with the highest bonding preference, meaning the one that makes the most bonds. For example, comparing nitrogen and oxygen, nitrogen wins because it likes to have 3 bonds while oxygen prefers 2. You propose what's called a sigma bond framework. 'Propose' implies that this process will take some trial and error. You might not get it right on the first try, so it's advised to initially write something that might seem incorrect, just to start the refining process.
If two atoms have the same bonding preference, such as sulfur and oxygen, you should place the larger one in the center, meaning here sulfur would be chosen over oxygen. Then, you complete all the octets using lone pairs, filling in the structure with lone pairs everywhere to complete everyone's octet. The next step involves a bit of math where you calculate the theoretical valence electrons, calculate the actual number of electrons observed, and then subtract the two numbers to find the electron difference. If there's a positive electron difference, then you create double bonds; if there are too few dots, then add more lone pairs to meet the expected theoretical valence.
However, with the understanding of organic chemistry and knowledge of bonding preferences and formal charges, you can now utilize these to determine the structure without repeatedly performing the previously mentioned calculations. Thus, steps relating to electron calculation become significantly streamlined as we apply organic chemistry principles.