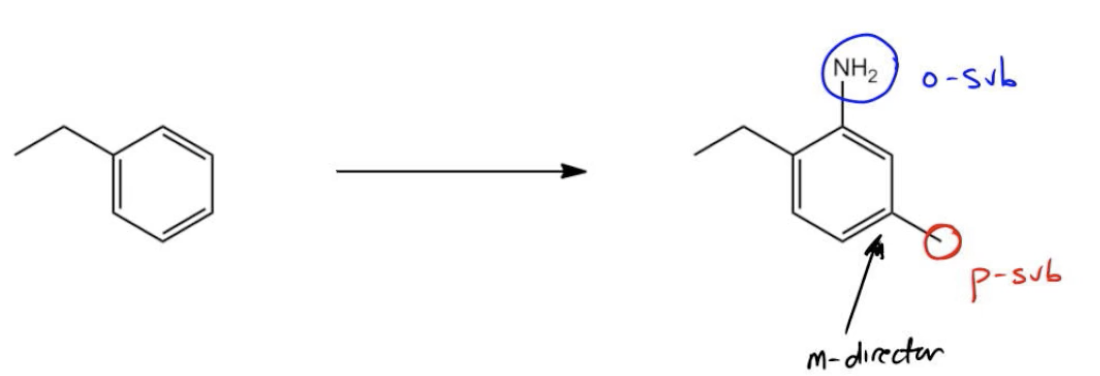
All right guys, there was a lot going on in this question. Notice that pretty much the only thing that stayed the same was the ethyl group. But a lot of other stuff changed. First of all, I'm going to need to find a way to put a methyl group in the para position. This is para substitution. Even harder than that, I'm going to have to put an aniline in the ortho position, so ortho substitution. Now we've got a few issues here. First of all, is ortho substitution very favored? In most cases, no. The only way that I'm going to get a high yield of the ortho position is to make sure that this methyl group is there first or something is there blocking the para position. I need to block it so that it's going to be forced into the ortho. On top of that, ideally, this should be a meta director when I add the aniline because I want it to be synergistic with the ethyl group to face towards the top. I should be thinking, are there any groups that I could add that could be a meta director and then be turned into an ortho para director later? That's one thing. Another thing is do we even know how to add aniline to a benzene? Did I ever teach you how to add NH2 specifically to a benzene, an EAS reaction? No. But did I teach you a precursor that can be easily turned into aniline? Yes guys. Remember, reduction of nitro groups. We can use nitration to make aniline which is why it's so popular. So at some point, I need to nitrate that position. But only after I already have some kind of meta director in this position. See where this is going? You might not, but let me just step in and try to help. This is just you have to just start getting a feel for these.
The first reaction we're going to do is a Friedel Crafts acylation because we know that acylation can be turned later on into alkylation using a Clemmensen reduction. I'm going to use an acid chloride. Now this part is important guys. Your acid chloride needs to contain the number of carbons that you want in your end product. Since I'm adding it here, how many carbons should this acid chloride have? Just one because I only have one carbon here. That means that I should have an H on the other side because I only want one carbon total and that's the carbon on the carbonyl. I'm going to combine that with AlCl3 and I'm going to get a molecule that looks like this. I got an aldehyde for right now. Notice that it attached to the para position because we said that para is pretty much predominantly favored, especially when you have larger groups like acyl groups. Cool. Now what can we do? Well, now I have a meta director here and an ortho para director here, now is the time to nitrate because they're both synergistically pointing to that top position. Now I would take my nitric acid and my sulfuric acid and I would now make my nitrated, my nitro group along with my aldehyde at the bottom.