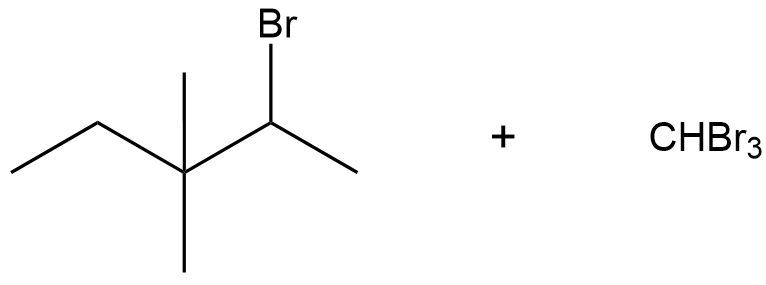
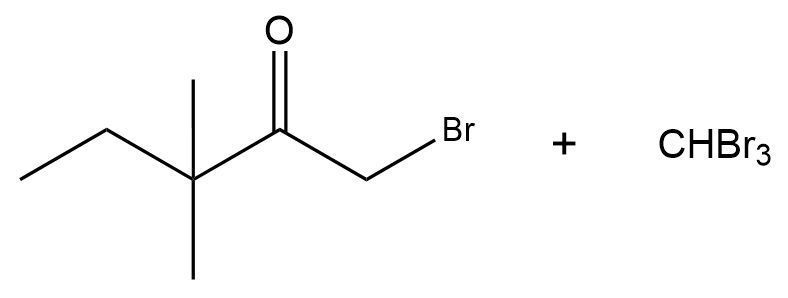
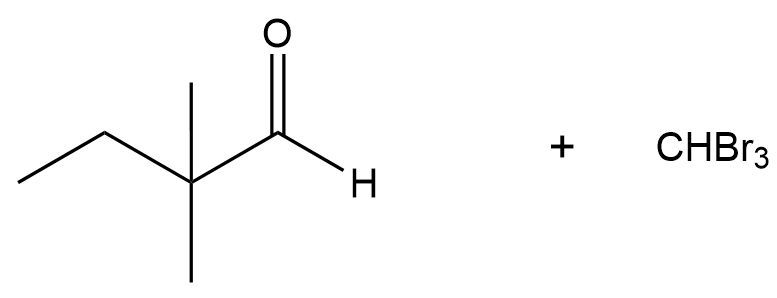
The haloform reaction is simply a base-catalyzed alpha halogenation of methylketones. It's the same thing as base-catalyzed alpha halogenation except that you specifically have a methylketone. Let me show you the mechanism. The reagents are just the same as we were dealing with before. The only difference being that I'm starting with at least one methyl group on one side. What's going to happen is after the formation of your enolate and attacking the halogens, you're going to get a polyhalogenated alpha carbon. Makes sense so far?
But here's the deal. If you have a methylketone, you've now made an awesome leaving group because you have CX3. The next equivalent of base that reacts, instead of just sitting around and thinking, "hey, I've got nothing else to halogenate, I'm done," can actually go through an SN2 mechanism and it can form a tetrahedral intermediate. You would get a tetrahedral intermediate that looks like this: O-, R CXXX (X). Carbon is usually not a good leaving group, but a carbon with 3 halogens is an extremely good leaving group. Then I would kick out the CX3.
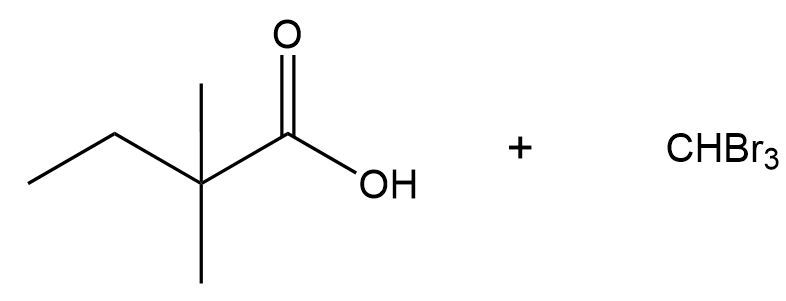
That's going to give me a carboxylic acid. Crazy, right? It's going to give me carboxylic acid plus it's going to give me CX3-. Now I know my head's in the way a little bit. But then to end this reaction, the CX3- is going to deprotonate the acid because it's an acid. You're going to wind up getting a carboxylate. And the molecule that the reaction is named for, haloform. Haloform is just basically a methyl group with 3 hydrogens replaced with halogens.
The entire idea behind haloform is that the alpha carbon of this reaction is transformed into a good leaving group through successive halogenations. Eventually, the O- just kicks it out entirely, which is something that would not happen in a base-catalyzed mechanism without it because if a base were to attack here, it would have nowhere to go because it has nothing to kick out. It does not have a good leaving group. But in this reaction, it does have a good leaving group which is why you get the final products.
Just extra facts, haloform depends on what you're using. If it was chlorine, then it would be chloroform. If it's iodine, then it would be iodoform. Some of these end up being tests that you actually use in your chemistry lab to test for methylketones. A lot of times, iodoform will precipitate out of the solution and it's like a yellow precipitate, and it's a test to see if you had a methylketone because if you have that precipitate, that means that you formed iodoform which means that you had a methyl group on your ketone. Interesting, right? Cool, guys. Let's move on to the next reaction.