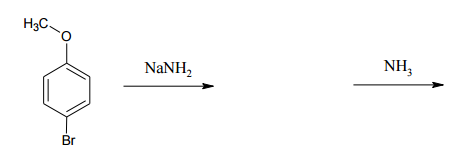At this point, we've learned about 2 different addition elimination mechanisms for benzene. One is electrophilic and one is nucleophilic. But it turns out that there's another form of nucleophilic substitution out there. But this one is an elimination then addition pathway. It's weird. This is called the benzyne pathway. It turns out that benzene can progress through an elimination addition pathway. But it's going to make a very unstable intermediate. That unstable intermediate is called an aryne or benzyne because it literally has a triple bond within the ring. Imagine how unstable it is to have a triple bond. Remember, triple bonds want to have a bond angle of 180 degrees. It's going to be forced into a 120-degree bond angle on that cyclohexane. It's going to suck. But it can happen and this is actually a way that we make amylenes.
Let me show you guys the kinds of mechanisms that we've learned so far and how benzene is similar and how it's different. It's mostly just different. I'm going to go through this really fast. We know that for EAS, I can add first and then I can eliminate. We get our addition and elimination through an electrophile. We call it EAS. We know that for SNAr, I can also add first. But the way we add is actually a nucleophilic attack. And then we eliminate using the same anion. We also get additional elimination but this one is nucleophilic, so SNAr. The aryne pathway or the benzyne pathway is much different because what happens in the benzyne pathway is that we have to actually eliminate first. How do you eliminate from benzene? You literally have to perform an E2. You literally have to do a beta elimination on this benzene. You would grab one of the H's with a strong base. You use a strong base and you would literally do the 3 arrows for an E2 attack. I would grab the H, make a triple bond, and kick out the X.
What this is going to make is a very unstable benzyne, also called aryne because arene is a benzene ring, so a benzyne intermediate. Extremely unstable. Now we have to add. The way we add is with the conjugate base or the conjugate acid. The conjugate acid now can be used to add to the benzyne. The mechanism is a little weird but just bear with me. What we are going to do is we are going to take our conjugate acid and we are going to attack one side of the triple bond and kick those electrons down to form an anion. What this is going to form is another intermediate that looks like this: I've got my benzene back now with a negative charge. Then I've got B H where now B has a positive charge because that has one too many bonds. To finish this up, this negative can do an intra basically a proton exchange. It can finally grab the H and we can get our substitution. That substitution, it took a long time. It's a kind of weird pathway. But we got a base to switch out for a halogen.
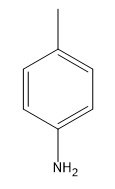
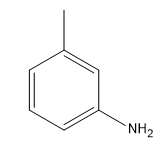
What I wanted to show you guys is how we can use this to make an aniline. How could we make an aniline using this mechanism? The base that we use, this is a very typical reaction, is NH 2-, a very strong base. We are going to do an elimination first. We are going to take an H and we are going to do a beta elimination. This is going to give me my benzyne. Cool? There's my benzyne. Awesome. What happens next? We'll notice that now I actually have my base is NH3 and I still have a lone pair. It's still somewhat nucleophilic. I can use the conjugate to attack or add to one side and kick electrons down to that side of the double bond. What I'm now going to get is a molecule that looks like this. Oops. I want to keep the double bonds in the same place. NH let me put the H's on the other side. NH2H positive and negative charge. Then we do the proton exchange and we're finally going to get what? What you just finally get at the end of this is aniline. This is actually a way to make aniline. You can make aniline using an NH 2- base on basically an aryl halide. You start off with an aryl halide plus NH 2- can give us aniline. Crazy, awesome. Let's go ahead and flip the page.