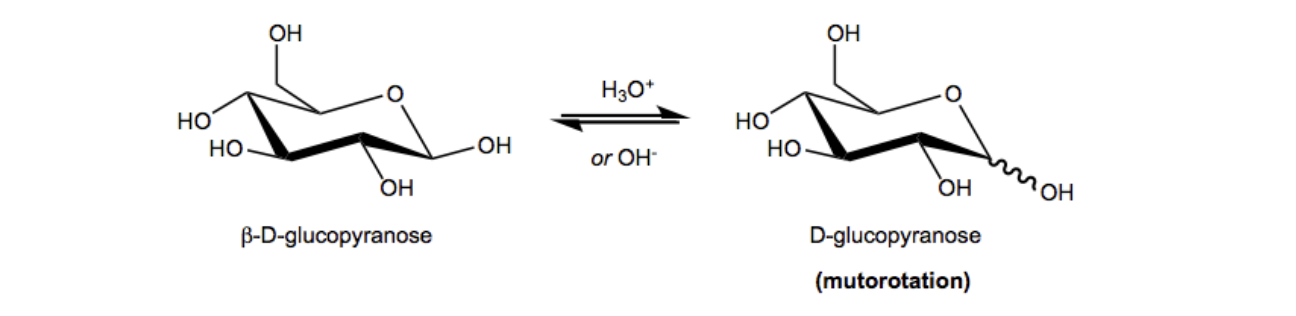
Now I want to dive deeper into the process by which an alpha anomer can become a beta anomer and so forth. So guys, pyranose and furanose rings are constantly hydrolyzing back and forth between their cyclic and straight-chain forms. Now you guys know what that mechanism looks like, you know what the rings look like, and I'm here to tell you that it's in constant equilibrium. It's not just like one ring gets formed then it stays like that; it's a constant equilibrium of hydrolysis, cyclization, decyclization, etc. Okay. Well, the specific process by which an anomeric position would convert between the alpha and beta forms is called mutarotation. It's basically the backward reaction of cyclization, but it's so that you can achieve the other anomer, okay? Now here in this example what I've done is I'm showing you how let's say you start off with a beta-D-glucopyranose and you expose it to either acid or to base, just a little bit of acid or just a little bit of base, this ring is going to start, is going to begin to hydrolyze and it's going to turn to the straight chain, and then it's going to eventually mutorotate forming the other anomer. Now what I did in this drawing is I didn't draw just the alpha anomer, I actually drew this squiggly line. What does that squiggly line mean? It means that you lose the alpha and beta anomer information because you're going to get some mixture of both. Now it's important to note, this is not going to be a racemate. Remember what racemate means? It means that you're going to get 50 of 50 of each. I'm not saying that, that's not what the squiggly line means here. It just means you're going to get a combination. So here instead of putting beta-D-glucopyranose, I just put D-glucopyranose because you're going to get a mixture of the two. Mutarotation is going to make it so that you're just going to have some kind of mixture of the two.
So guys, it turns out that in your textbook, the explanation of mutarotation is very, very closely tied with this idea of optical activity. So what I want to do is instead of having you read that and be confused, I just want to explain it so you can understand why they always talk about optical activity when they talk about mutarotation. And what it is is that optical activity provided the proof for mutarotation, which I'm going to show you now. So first of all, it's important to note that anomers always differ in optical activity. Okay. You're always looking α anomer is always going to rotate light differently than a β anomer, okay. For example, the alpha anomer of D-glucopyranose rotates light at a positive 112 rotation in a polarimeter okay. Whereas a beta-D-glucopyranose rotates light at around positive 19. Okay, guys these are super random numbers that you do not need to memorize, Okay? But this just shows you the completely different optical activity of the alpha versus the beta. Now if you weren't paying attention and maybe you just kind of thought very quickly, you might think, you might wonder why are they not just opposite rotations. Because remember that back when we talked about optical activity a long time ago, we talked about how if one enantiomer shows the positive rotation, the opposite enantiomer should then show the negative rotation of that same rotation of plain polarized light. In this case, they're both positive and they're very different from each other. Why is that? Well, guys, remember that anomers are not enantiomers of each other. Right? They're not enantiomers. Like a bunch of the chiral centers stayed the same. What they actually are is remember they're diastereomers. Okay. So that's why they have unrelated activities. You can't predict that one is going to be positive and one is going to be negative. They just have random numbers. Here I've given you the random numbers for glucopyranose, pyranose, but other types of rings, you would need to actually use a polarimeter to get that number. Okay? So it's not something that you're supposed to memorize.
Now why is this important? Well because back in the day when they were first discovering kind of this relationship between optical activity and chirality, it was noticed that D-glucopyranose always equilibrated to a positive 52.5 rotation, which is weird because that's not the rotation of either one of these right? 112 is one, the other one is 18.7 and somehow it would always rest if you let it sit around long enough, no matter which one you started with. Whether you started with 100% of the A or whether you started off with 100% of the B, they would always end up at a positive 52.5. Now this is strange because this is not even the midpoint, this is not even the halfway point of rotation between the two optical activities. So what I actually did is I made a little graph for you guys so you guys can really understand this. So notice that the beta had a rotation of positive 18 right and the alpha had a rotation of positive 112 right? So that means at the very beginning, let's say that we started off with a 100% of the beta anomer. Well, that would give us 18.7 optical activity. But what was noticed is that after several days of it being sitting in an aqueous solution, the optical activity would get more and more and more positive until it reached this magical number of 52.5. Now why is that? And then the same thing happened with this guy, with the alpha. The alpha would get lower and lower until it got to 52.5. Well, guys, the reason is because we learned, remember that we learned that specifically for beta-glucopyranose, the beta prevails at a 64% equilibrium and the alpha prevails at a 36% equilibrium, meaning that if you were to just start with one of them and allow it to mutarotate, you're eventually going to reach an equilibrium where 64% of the pyranose is in the beta form and 36% is in the alpha form. And that is why at the end, the rotation that you end up getting is 52.5 because that happens to be right at the 64% mark of the difference between the two. What this shows is that 64% of it is the beta and only 36% of it is the alpha. If it had been 50/50, let's say that it equilibrated to exactly 50/50, then we would expect a rotation right in the middle of these two, which is positive 65, but we don't see positive 65. What we see is that it's a little bit more than 60, it's a little bit less than 65, which must mean that the one that's on that side is the majority because it's the one with the lower optical activity and when you actually do the numbers, it shows that 64% is equal to the beta. Okay? So anyway, this is proof that mutarotation was occurring. You know, chemists back in the day, they realized oh, this must mean that, this must mean that if I have an alpha somehow it's going to equilibrate and become a beta. So then they started figuring out well what is a potential mechanism for this to take place? How could an alpha potentially become a beta? Well, that's going to be the next video. So hopefully this makes sense so far in terms of optical activity proving that mutarotation exists, and in the next video, I want to go into the mechanism.