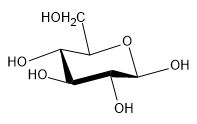
Now it's time to dive deep on the most important reaction of monosaccharides, and that is cyclization. So guys, it turns out that in aqueous solutions, monosaccharides are most stable in their cyclic form. Monosaccharides can form stable 5 and 6 membered rings, which have different names. A 5-carbon cyclic sugar is going to be called a furanose, and a 6-carbon cyclic sugar is going to be called a pyranose. There are no 4-carbon cyclic sugars or 7 and greater because those aren't stable as rings anymore. So, really the only stable ones that you have to worry about are furanose and pyranose. Cool? The nucleophilic addition of the penultimate alcohol to the electrophilic carbonyl leads to cyclization. It doesn't always have to be the penultimate alcohol, but usually, it is. Let me just kind of show you guys what we're talking about here. This is D-glucose, as you guys should be well aware of. Here is the penultimate alcohol and the electrophilic carbon. Remember, it has a partial positive charge. The nucleophilic addition, meaning the O comes in, attacks, and kicks electrons up to the O; that reaction right there is going to cyclize this straight chain glucose into one of 2 possible rings.
Now, why would you get 2 possible rings? Well, the carbonyl carbon, if you think about the geometry of a double bond of an sp2 hybridized carbon, it's trigonal planar. That means that it can be attacked either from the top or from the bottom. The oxygen could either come around the top and attack or around the bottom and attack. It turns out that this is going to set up for stereoisomers that are possible. When these monosaccharides cyclize, 2 different C1 epimers are possible. The way that monosaccharides are numbered is always from the one carbon being the point of attack being the carbonyl because that's the top carbon. This is C2, C3, C4, C5, and C6. After cyclization, this becomes carbon 1, carbon 2, carbon 3, carbon 4, carbon 5, and this is actually carbon 6 right here. The oxygen doesn't get a number because it's not a carbon. When I say that cyclization leads to 2 different C1 epimers, what I'm saying is that you're going to have 2 different chiralities at carbon 1 that are possible, and these 2 different epimers are actually specifically known as anomers.
The specific epimer at the C1 position, the two different positions are called anomers. What we have is the alpha anomer, that's possible, and the beta anomer, that's possible. The alpha anomer for specifically with D-glucose, the alpha anomer is the one that faces the O down, meaning that the O must have attacked from the top because it attacked from the top and pushed the O down. The beta anomer faces the O up, which means that the carbonyl must have been attacked from the bottom so that it would push this O up. This explanation lies in equatorial preference. Do you guys remember the principle of equatorial preference in cyclohexane? And how larger groups like to be equatorial. See, how my O is equatorial? That is going to be part of the reason that this percentage is higher than that one because it likes to exist in the equatorial form. But guys, as much as I wish that was always true, there are exceptions to this and there are going to be some sugars that actually prefer the alpha anomer for other reasons.
The nomenclature has gotten significantly more complicated. A D-glucose with the penultimate alcohol facing towards the right, but once it cyclizes, it makes a 6 membered ring, therefore, D-glucose turns into D-Glucopyranose because this stands for the 6-membered ring. The beta is determined from the anomer. The system is simple: if the OH on a carbon in the Fischer projection of your monosaccharide faces right, it's going to point down in the ring; if it faces left, it will face up in the ring. Your last one, the penultimate one, the stereo descriptor does not follow that rule. If it's a D sugar, it's going to face up on the ring. If it had been an L glucose, then it would face down.