In this video, I'm going to walk you guys through a technique that you might need to use for Diels Alder problems. Sometimes your professor, your textbook, or your online homework is going to ask you to do a Diels Alder retro synthesis. That means that you're going to be given the final cyclization product and then you're going to be asked which diene and which dienophile were required to make this 6 member ring in the first place. Now it turns out this is a really easy type of question to answer if you just have the right technique and that's what I'm here to show you. Here's an example. Which diene and dienophile would you use to synthesize the following compound? It's really easy to get lost in these types of questions because as college students, we hate doing stuff that we haven't been taught to do explicitly, right? And this is a backwards question. You have to think backwards. So I'm going to teach you guys how to think. I already taught you guys how to think forwards and I'm going to teach you how to think backwards as well.
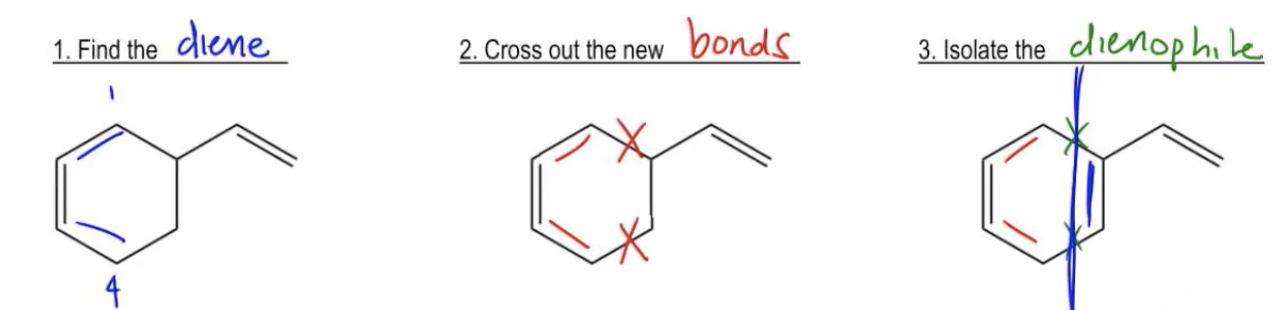
The first thing that you guys want to notice about this product is that there's a certain kind of landmark that you're always going to be able to find on these products because you know about the mechanism. And the mechanism, remember, always makes a double bond between the second and the third carbon. Remember me showing you guys that? So that means the first thing you want to do is you want to find that landmark and you want to orient yourself on that because that's going to provide the structure for the rest of the molecule. The first thing I want to do is identify where was that new double bond. That new double bond is right here. That means that this must have been my second and my third carbon, which means that that must have been where my diene was. My first step is always to find the original diene. The way you do that is by identifying the double bond and then saying well, this must have been the diene to begin with. This must have been my 1 and this must have been my 4. Now that I have that diene, I understand the mechanism better. I know that the diene must have reacted with a dienophile. Remember that the diene always creates 2 new bonds to the dienophile. The next step is going to be to cross out the new bonds because I know that my diene must have made 2 bonds.
Here's my diene once again and it made 2 new bonds. I'm going to cross these out because I know they didn't used to exist. So after I've crossed them out, that means that whatever is attached on the other side must have been the dienophile. So in my next step, I isolate the dienophile. And guess how you do that? Really easy. All you do is you take your x's, right? You still have your diene and you just cross a line right through them. So I'm going to just chop it off right there, right through the x's. And what we see is that now we have a diene on one side and something on the other. The other side must have had a double bond in order to react in the first place. Basically, we're making those 3 double bonds that we started with. So the answer for this question would be that I start off with a diene that looked like this, a 1,3-diene that looked like this Plus, I started off with a dienophile that looked like this. Okay? And now that we've figured that out, isn't it crazy to see how actually it was a dimerization? This is a molecule that reacted with itself through Diels Alder but it wouldn't have been obvious unless you use a system to figure it out. Okay? I know this is a new skill. I know that I might be making it look too easy. Let's test out our skills on this example. Go ahead for the following two problems. Try to figure them out. We'll do them 1 at a time. Go ahead and do A right now and then I'll help you guys and show you the answer.