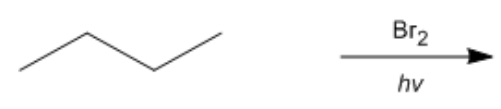Hey everyone, so up to this point we've learned that radical halogenation prefers to occur at the most stable radical cation intermediate. In most cases, that's the tertiary position. But in this video, we're going to learn that not all halogens are created equally and, in fact, some halogens at times choose a spot that's not always the best spot. Now, in order to think of this in everyday terms, just imagine you've been invited to the greatest steakhouse in the world where it has all types of meat selections, whatever you could think of. Now, here we're going to attach this idea of our steakhouse to the different types of meat entrees you select and we're going to say that these meat entrees represent different types of halogenation reactions. And the more exothermic your halogenation reaction, then the more non-selective it is. It basically halogenates anywhere on your organic compound and therefore the more unhealthy it is. Again, playing along with this whole idea of food selection. Now, this video is just taking a look at qualitative radical selectivity and we're going to go into greater detail later on and try to connect the idea of exothermic reactions and selectivity. Now, first of all, what exactly is radical selectivity? Well, selectivity is defined as the ability for radicals to only halogenate the carbons with the most stable radical intermediates.
Again, we're going to learn that not all halogens play by the rules when it comes to this idea. Alright. So here we have 4 individuals that have been invited to this awesome steakhouse, the greatest in the world. The first one is not really picky in terms of food. They'll eat whatever. They are not selective whatsoever, and they choose a typical hot dog. Okay. We're going to say that this hot dog, which represents a highly exothermic reaction, also represents fluorination. Here, fluorination has an enthalpy value that is negative 432, so really negative ∆H. So it's very nonselective, very unhealthy because we know hot dogs are made up of who knows what tons of different parts of animals. And we're going to say here, that fluorination, because it's not very selective, because this diner doesn't really care what they're eating, it is not useful in terms of, radical halogenation. So when we say fluorination, we have F2 and we have ultraviolet light, we're going to say it's a no-go. It's too exothermic, too uncertain. We don't know what it's going to do. It's so negative, in fact, that in some cases we can deem that as being an explosive reaction. So we want to stay clear away from fluorination, and let's move on to our second person.
So here, the second person, is not willing to eat whatever. They're kind of stuck in their ways. They only want to select something that they've eaten before. So they're gonna play it safe. They're gonna choose meatloaf and other dishes that they're familiar with. If we try to connect this to chlorination, we're going to say that the overall enthalpy value of chlorination is negative 101. Now, here it's still exothermic because it's negative, but it's not as negative as fluorination. Because it's exothermic, it's still a spontaneous reaction which is good. But the issue is it's still just a little bit too negative. As a result of this, chlorination, we're gonna say the only useful radical chlorinations are reactions with a single type of hydrogen. Meaning that if I choose an organic compound that has different types of hydrogens: primary, secondary, tertiary, chlorination will give me a mixture of products. It's not very selective. It'll do a little bit of here, a little bit of there. Just like our patron at this restaurant, they're only going to stick to the foods that they know. And they've eaten quite a few things.
Now we have our 3rd person. This person really knows their different types of meat selections, and they know exactly what they want. They want the most expensive. They want the highest quality cut in terms of this. Here, they want maybe Wagyu beef or something, something they see that as being the most expensive. So this, we relate to bromination. Bromination has an enthalpy value that's negative 26. So it's still exothermic, not quite as exothermic as fluorination and chlorination, But since it's exothermic, it's still spontaneous. We're going to say here that bromination is the only useful method for selectively halogening alkanes because you can control what it'll brominate. So when it comes to bromination, if we have a mixture of tertiary, secondary, primary hydrogens, bromination always goes for the most stable radical cation intermediate that's presented. In most cases, tertiary is more stable than secondary, which is more stable than primary. Right? So we can count on bromine to brominate what we want it to do. So here we have Br2 HV, and depending on what we have it'll brominate in the place that we want.
And then finally, we have our 4th person. So we've come to the greatest steakhouse, greatest meat selection in the world, but then our 4th person says, I don't want any meat. I don't wanna get involved in any of this. So here, we're gonna relate this to iodination. Iodination has an enthalpy value that is positive 53. Because its enthalpy is positive, it's an endothermic reaction. Remember, endothermic reactions are nonspontaneous and it would require our investment of energy for it to go. So you'd have to basically, push this person to try anything. But again, they don't really want to do that. They don't want to get involved in any of this meat selection. Just like iodination doesn't want to get involved with any type of radical halogenation. So here again, it's nonspontaneous so it doesn't even want to get involved.
Now, finally we're going to say here, chiral products are always, racemized. So here we're talking about racemic mixtures. Now, what exactly does that mean? Well, if we think about it, this has to do with if our starting material belongs to a chiral center or not. So let's say that we had this organic compound and here we have a methyl group, and then here we have our hydrogen, here we have ethyl, and here we have propyl. And we did bromination. So bromination will want to replace this hydrogen here. Now that bromine can either come in from the front or the back of the molecule in order to replace that hydrogen. So because it has those two options we have 2 possible products. One where the bromine would be dashed, and one where it would be wedged. And it would just switch positions with the methyl. And you will get 50% of this one, and 50% of this one. So you create a racemic mixture. We have, 50% of both of these products. So that's what we mean by chiral products are always racemized. So again, we're looking at this qualitatively. We're trying to relate the whole type of selectivity associated with the halogen based on the preference of different patrons within the greatest steakhouse in the world. Right. So again, we're going to go into greater detail in terms of this from a qualitative to quantitative analysis later on. For now, this is just helping us to relate how the different halogens react when it comes to radical halogenation.