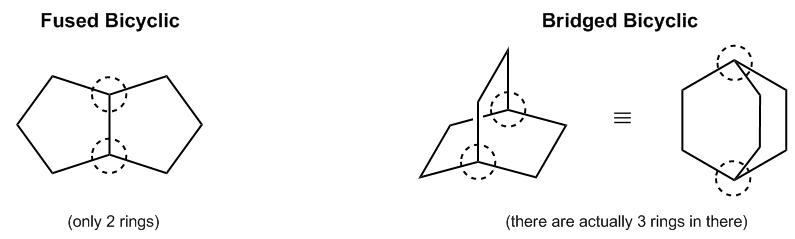
As we discussed earlier, bicyclics essentially fall into two different categories. There are what I refer to as the normal bicyclics, which is simply a structure consisting of two rings attached by a single bond. Imagine this as ring number 1 and this as ring number 2; this configuration represents a normal bicyclic.
Then, we have another type of bicyclic called the bridged bicyclics. These structures actually comprise three total rings. It may be challenging to visualize, but imagine having one ring at the base. A second ring is formed on one side, and then the third ring is formed on the opposite side. In this setup, you have three rings that are all part of this compound structure, interconnected by what are known as bridgehead atoms. Note that these are the atoms right here, referred to as bridgehead atoms.
Visualizing this might be difficult, but it turns out that both of these structures, the three-dimensional one drawn here and the other one over here, represent the same molecule; they are merely two different ways to depict the same molecular structure. Specifically, the lower part here represents a cyclohexane, shown in blue, and the bridge, which crosses over the top and is depicted in red, is right here. These drawings exemplify the two-dimensional or planar representation versus the three-dimensional representation. However, regardless of the depiction method, they are the same molecular entity.
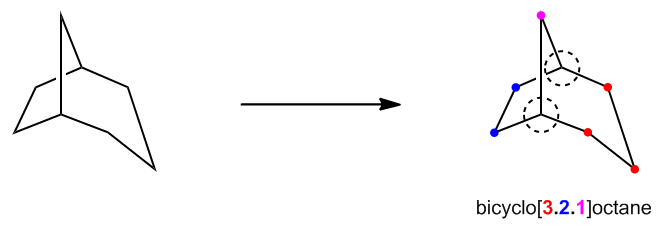
Let's proceed to examine how to name these compounds. Bicyclics, with their added complexity due to multiple rings, follow a distinct nomenclature system compared to monocycloalkanes.