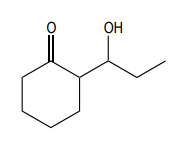
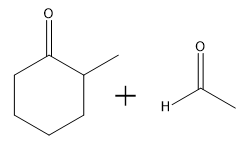
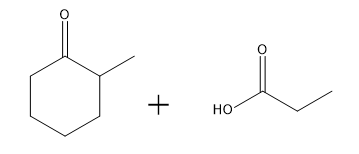
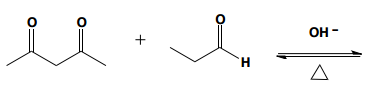
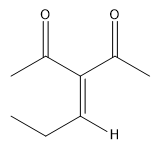
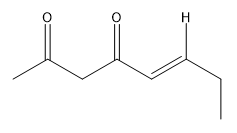
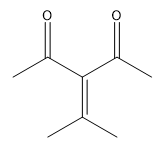
In this video, we're going to explore what happens when you try to run an aldol condensation on a mixture of different aldehydes. Whenever you run a condensation on 2 different ketones or 2 different aldehydes, mixed products are going to be difficult to avoid because what's going to happen is that we've got 2 different enolates that are possible, not within the same ketone or aldehyde, but on different aldehydes. That opens up a big can of worms because for example, let's say we have aldehyde A that can form an enolate here and aldehyde B that can form an enolate here. We've got an issue because it turns out that once you form enolate A, A can react with itself to give you A. A negative could attack A, the actual protonated version of A to give you A A negative could also attack B, so the non enolate version of B to give you a B condensation product. B negative, so the enolate of B could attack A to give you B A which by the way, AB and B A look like they're similar but they're not. They're actually going to be different compounds because you have different numbers of carbons in each. Finally, B negative could react with B to give you BB. What's going to happen? You're going to get this terrible Punnett square of aldol products and it's just going to be a disaster. For the sake of teaching purposes, I am going to fill in this square, so you guys can see all the possibilities. But typically, this is not something that we would ever do in a classroom. You would never draw all 4 aldol products because it's just it's a synthetically useless reaction. No one wants that high of a mixture of products. But for the sake of learning, let's just see what each of these combinations would look like.
Always remember that the first letter, I'm using as the enolate. I'm doing enolate, enolate, enolate, enolate. I'm doing my whole left right thing. Let's start off with AB. And I am going to you might need to gr