Equatorial preference has to do with the fact that one of the two positions, remember that there is the axial position and there is the equatorial position, one of them is going to be much more crowded or what we call torsionally strained than the other. Now, usually if you just have hydrogens in there, it's not a big deal. But if you start adding bulkier groups in there, it's actually going to affect it. Okay? So let's just look at the different positions. Remember, we have our axial positions, they're going straight up and down with the corners and remember that we have our equatorial positions going slightly opposite. Are you guys cool with that so far?
And now let's imagine that I put different shapes here. So let's say that I just put a bunch of maybe like green circles on the equatorial positions, and let's say that I put some blue circles on the axial positions. That sounds like it hurts. So, we have these ones on the positions and I just want to analyze the ones at the top. Let's just say that we look at this blue circle, this blue circle, and this blue circle versus this green circle, this green circle, and this green circle. Are you guys following so far?
In fact, let's go ahead. You don't have to do this, but I'm just going to erase the other ones so you guys don't get distracted. So you guys can really see what's going on here. See, so that's how clear I want it to be. So basically, we've got our axial positions and our equatorial positions. Which of these do you think is going to be the most spread out? And then which of them do you think is going to be the most tight together? And it turns out that the blue circles are really close together. Okay. So it's like awkward and stuff. They do not want to be there. Alright. On top of that, they're like sitting on sticks. It's terrible. So whereas the equatorial positions, they've got all this room to spread out. It's awesome. Look how far apart they are. Okay?
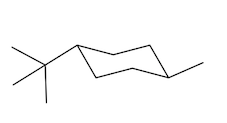
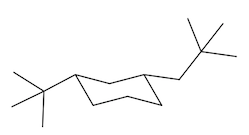
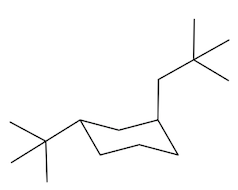
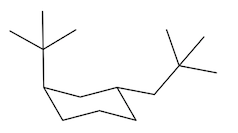
In fact, if you want to think about the equatorial position, it kind of looks like it's the equator of the earth. If this is like a big globe, the equatorial positions would be like on the equator, the axial positions would be like on the north pole. Alright? And the south pole. So you don't want to be stuck on the south pole or north pole. You want to be like in paradise, like on an island drinking a Corona. Okay? So the axial positions suck. That's what I'm trying to say. Especially when you put large groups there, you do not want to be in the axial position. So what that means is that the ring is always going to flip in order to accommodate the preference of the largest substituent. So, in this case, I have a tert-butyl group and that tert-butyl group can be on 2 different chairs. It could be on one chair that has it in the axial position, but anytime that you flip a chair, you wind up flipping positions. Okay? So if you flip your chair, you also wind up flipping positions. So now this would become equatorial over here. Alright? So it goes from axial to equatorial.
Which of these do you think is going to be the most stable? And it turns out that it's going to be way more stable in the equatorial position. In fact, over 99% of this compound is going to exist in the equatorial position, and less than 1% is going to exist in the axial position. Why? Because the axial is so much more torsionally strained with these hydrogens here. See, they're just bumping into each other. Whereas, the equatorial position is way better. Okay. So as I just said, when chairs flip, remember that axials are always going to become equatorial and equatorials become axial. Okay. So any time you flip, you're going to be giving something in the axial position an opportunity to become equatorial. But you also have to change the shape of the chair as well.