So far we've been learning about radical halogenation mechanisms and how the radical is always going to be attracted to the spot that has the most r groups. Okay? So the tertiary is going to be more stable than primary. But what we also know is that according to the radical stability trend, allylic radicals are the most stable. Okay? So now I want to throw that extra complexity into the mix. What happens if there are allylic positions available to be halogenated? And that brings us to allylic halogenation. So once again, I just want to show you guys, just in case you forgot, that the allylic and benzylic radicals are more stable than primary, secondary, or tertiary. So what that means is if I have one of these two possibilities, this is going to be my preferred spot, not those 3. Okay? Now, what's interesting about these is that these radicals can resonate. Okay? So what that means is that resonance is going to have to play a central role in this mechanism. We can't just ignore resonance. We're actually going to have to embrace it for this mechanism. So I just want to show you guys what this could look like.
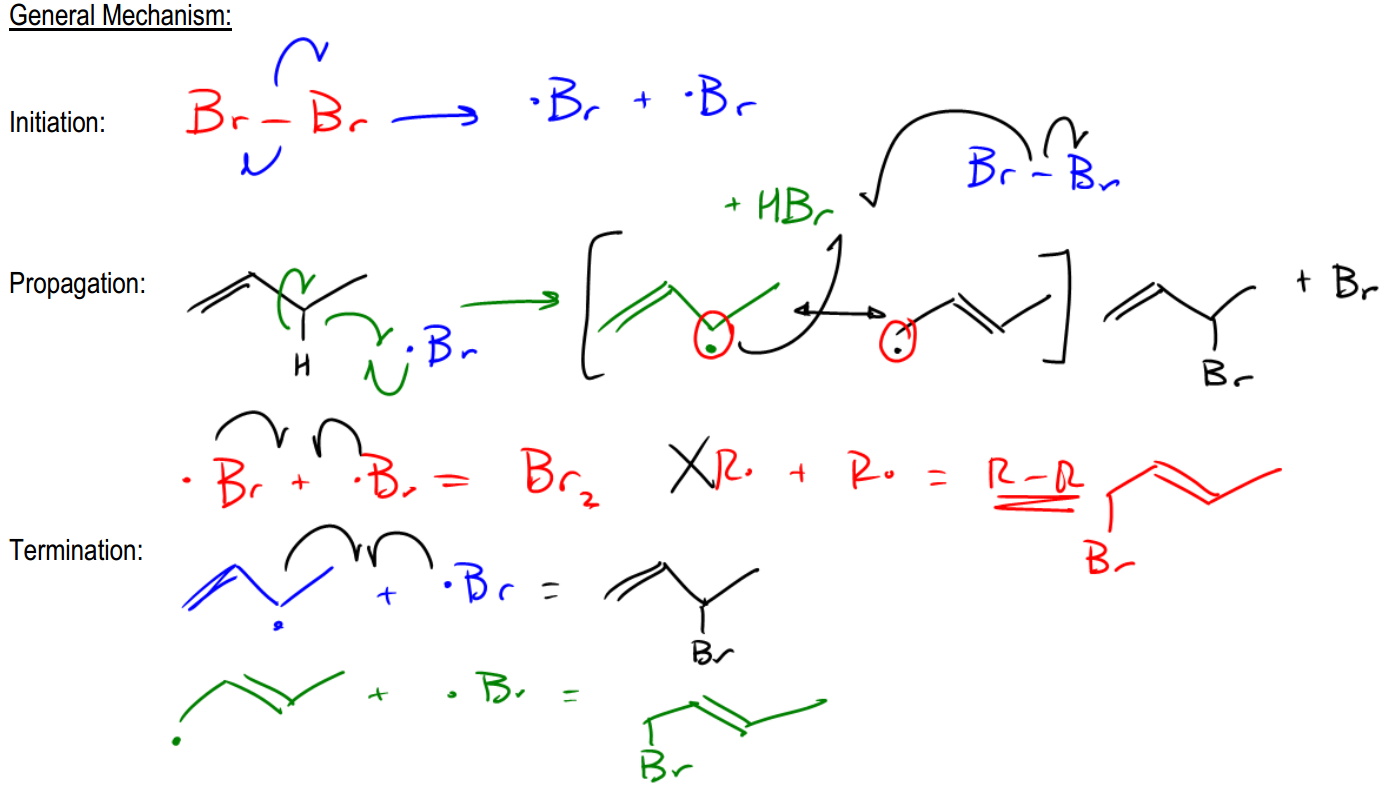
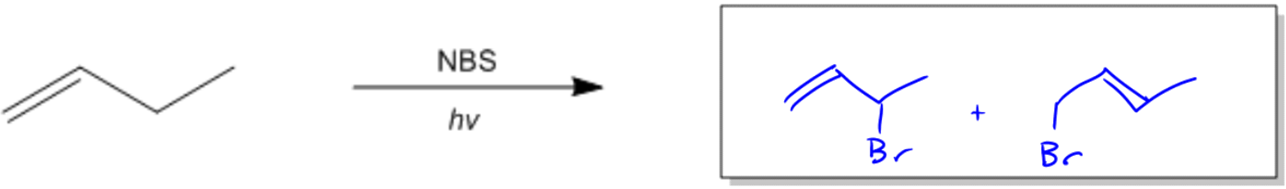
So basically, I've got a diatomic halogen. Let's say this is Br2. Okay? So it's selective. Right? And we've got a double bond. Okay? Well, usually we would just say secondary or primary. We're going to go for the secondary spot. But in this case, I actually have an allylic position. That allylic position is right here. Okay? Because that's the one next to a double bond. So that's actually going to be the position that's the most stable and that's going to be the one that I want to react with. Now let's go ahead and draw the whole mechanism out and I'm going to show you guys how things are a little bit different now.
So first of all, my initiation step is really easy. We're just going to use Br2. And where do those first arrows go? You would just make radicals. There and there. So now what I'm going to get is Br radical plus Br radical. Alright. So that's it for our initiation. We have our target radical. Now what we have is the propagation step. In the propagation step, I'm going to want to pull off the hydrogen that's going to give me the most stable radical, so obviously it should be this one. Right? So let's go ahead and react that. I would use 3 arrows. I would do 1 into the middle of nowhere, 1 into the middle of nowhere, and then one right there. Okay? So what I wind up getting is I wind up getting a radical that looks like this. Okay? Now usually, and also we would get HBr, right? Okay. Now usually what would happen is that my propagation phase would continue and I would have to regenerate the Br radical by reacting with Br2. Okay, so that's normal. That's what we're expecting. But wait, there's a little difference. This radical can now resonate. So what that means is that I can't actually just continue from here. I need to draw the resonance structure of this radical before it can continue. So what that means is that the resonance structure actually looks like this. Okay? And what that means is that now my Br that's going to continue in the propagation stage can attach both there and there. So it's going to be able to attack both of those carbons because the radical is hybridized between those 2. Remember that resonance structures, it's not an equilibrium. It's a mathematical like statistical average of where that radical is. So what that means is that I can go ahead and draw my mechanism with the Br here and I can draw it from the first one. Just to keep things easy, I'll just do this, there, there, and there. Okay?
And what I wind up getting is I'm going to wind up getting a Br here plus Br radical. Okay? But I'm also going to get something else. I'm going to get a mixture of products because the radical on the end could have also attacked. So what that means is that I'm also going to get a product that looks like this. Okay? And this is one of the complications of allylic halogenation. If your double bond isn't perfectly symmetrical, you're going to wind up getting a combination of products. And really, we don't deal with major and minor in this section. We're just going to say, hey, we're going to get both of them. Okay? So it kinda sucks. Alright?
So then finally, what would the termination step look like? Well, there are a lot of terminations. We could get a Br and a Br. Okay? We could get just any of these radicals. I mean, any of these that plus might be r. Okay. Sorry. I'm just going to draw this and this. Okay. And that would give us our product that looked like this. This. And we would also get the other one, the other radical that would look like this. The radical here plus Br. And that would give us the other looking product. Okay? Now, I know that there was another one that was the 2 r groups. That is almost never seen in here. But if you wanted to include it, you could just add like R radical R. Okay? And just say that that's going to give you RR. Okay? But honestly, like for these reactions, usually professors just neglect this because it's assumed that you're not really going to get much of this at all. Okay? What is important is that now you can detect when you're going to get just one product or when you're going to get a mixture. And when you're going to get a mixture is when you're doing an allylic halogenation. If it was just tertiary or secondary, you would only get 1. But since it's allylic, that means it can resonate. Okay? In this case, you would get 2 different structures. The way to know how many structures you're going to get is just to draw the resonant structures yourself. Okay? So now what I want to do is go more into depth on allylic halogenation with the specific reagents that we're going to use.