Hey everyone. I'm about to take you on a journey through one of the oldest and most elegant fields of organic chemistry and that's the field of sugar chemistry, also known as carbohydrate chemistry. So let's go ahead and get started. Alright guys, so before we even really get started I need to tell you one thing which is that sugars, saccharides, and carbohydrates are all different words for the same types of molecules. So I'm going to be using those words interchangeably throughout this course and you should just think whenever you hear sugar, saccharide, or carbohydrate, those are all describing the same types of molecules.
Now one of those words that you might say often but not really think about too much is carbohydrate. We talk about carbohydrates and nutrition all the time, but when you break the word down to its roots, what you see is that it's actually a hydrate of carbon. That's literally what the word breaks down to, what it's symbolizing. And in chemistry, we know that a hydrate is any atom or molecule that's combined with water. So when we say that a sugar is a carbohydrate, what we're saying is that a sugar is a carbon that's been combined with water.
Okay. Now there are many sugars out there, but the most basic unit of sugar is called a monosaccharide. So we're going to be spending a lot of time in this course, just talking about the properties of monosaccharides before we expand that further into larger sugars. All unmodified monosaccharides have the same general formula. No matter what their shape, what their size, they always have the same formula, which is carbon, H2, and oxygen all to the n, where n is a number greater than or equal to 3.
C H 2 O nOkay? So let's break that down for a second, a few words at a time. So why did I say unmodified? Unmodified because I'm saying this is the way the sugar begins its life, okay? You could always change a sugar to have other atoms on it later, but when it starts off, it's always going to start off as CH2O to the n. Now when you look at this general formula, it describes exactly what I just said about a carbohydrate, which is that a carbohydrate is literally going to be one carbon that's been combined with one water molecule. So it's cool how it actually breaks down definitionally like that.
Now the last thing is that where n is equal to 3 or more, all that's saying is that your smallest possible sugar is a 3 carbon sugar. It can be as big as you want, but it has to be at least 3 carbons. If it's less than 3, if it's 2, it doesn't really count as a sugar anymore because it just has a very different reactivity. It doesn't behave like the other sugars do. So we wouldn't call a 2 carbon molecule usually a sugar.
Monosaccharides can be represented as either straight chains or as rings. So you're going to see that throughout this section I'm going to be showing them to you as straight chains and then other times you're going to see them as rings, and we're going to explain more why they cyclize.
Now something you should know is that by definition a carbohydrate needs to have 1 oxygen attached to every carbon to be a carbohydrate. You can't have 2 oxygens on 1 carbon and then 0 on the other; that would not be a carbohydrate. Now remember guys, what does IHD mean? It meant that you're missing hydrogens in order to be a saturated molecule. And remember that an IHD could either come from a double bond or a ring, right? Not necessarily a 6 member, but a ring. So that makes sense according to my fact about that they could either be straight chains or rings. That means when it's a ring, it has the 1 IHD from the ring, right? And then when it's a straight chain, it must have the 1 IHD from a double bond.
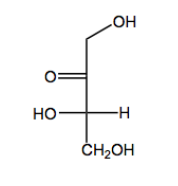
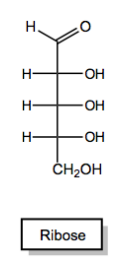
Monosaccharides always begin as either aldehydes or ketones. Once again, I'm using this word begin because you could always modify that functional group later. If you wanted to maybe could change the aldehyde into something else, but what I'm saying is that when they begin their life, they always start off as either an aldehyde or a ketone. Those are your only two possibilities. We call an aldehyde sugar an aldose and a ketone sugar a ketose. Let's look at two examples of monosaccharides.