 Back
BackProblem 1
Two solids are shown below. One is a semiconductor and one is an insulator. Which one is which? Explain your reasoning.

Problem 2
For each of the two-dimensional structures shown here (a) draw the unit cell (i)

(ii)
Problem 3
Shown here are sketches of two processes. Which of the processes refers to the ductility of metals and which refers to malleability of metals? (a)

(b)
Problem 5b
(b) What is the coordination number of each cannonball in the interior of the stack?

Problem 6
Which arrangement of cations (yellow) and anions (blue) in a lattice is the more stable? Explain your reasoning. (a)

(b)
Problem 7
Which of these molecular fragments would you expect to be more likely to give rise to electrical conductivity? Explain your reasoning. (a)

(b)
Problem 8c
The electronic structure of a doped semiconductor is shown here. (c) Which region of the diagram represents the band gap?

Problem 9b
Shown here are cartoons of two different polymers. Which one would have the higher melting point?

Problem 10a
The accompanying image shows photoluminescence from four different samples of CdTe nanocrystals, each embedded in a polymer matrix. The photoluminescence occurs because the samples are being irradiated by a UV light source. The nanocrystals in each vial have different average sizes. The sizes are 4.0, 3.5, 3.2, and 2.8 nm. (a) Which vial contains the 4.0-nm nanocrystals?
- Covalent bonding occurs in both molecular and covalent-network solids. Which of the following statements best explains why these two kinds of solids differ so greatly in their hardness and melting points? (a) The molecules in molecular solids have stronger covalent bonding than covalent-network solids do. (b) The molecules in molecular solids are held together by weak intermolecular interactions. (c) The atoms in covalent-network solids are more polarizable than those in molecular solids. (d) Molecular solids are denser than covalent-network solids.
Problem 11
Problem 12a
Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (a) Is Si a molecular, metallic, ionic, or covalent-network solid?
Problem 12b
Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (b) Silicon readily reacts to form silicon dioxide, SiO2, which is quite hard and is insoluble in water. Is SiO2 most likely a molecular, metallic, ionic, or covalent-network solid?
Problem 13a
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (a) molecular crystals?
Problem 13d
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (d) and metallic crystals?
Problem 14a
Which type (or types) of crystalline solid is characterized by each of the following? (a) High mobility of electrons throughout the solid;
Problem 14b
Which type (or types) of crystalline solid is characterized by each of the following? (b) softness, relatively low melting point;
Problem 14c
Which type (or types) of crystalline solid is characterized by each of the following? (c) high melting point and poor electrical conductivity;
Problem 14d
Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.
Problem 15c
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
- Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (a) InAs, (b) MgO, (c) HgS, (d) In, (e) HBr.
Problem 16
Problem 17
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
- You are given a white substance that melts at 100 °C. The substance is soluble in water. Neither the solid nor the solution is a conductor of electricity. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
Problem 18
Problem 19a
(a) Draw a picture that represents a crystalline solid at the atomic level.
Problem 19b
(b) Now draw a picture that represents an amorphous solid at the atomic level.
Problem 21b
Two patterns of packing for two different circles of the same size are shown here. For each structure (b) determine the angle between the lattice vectors, g, and determine whether the lattice vectors are of the same length or of different lengths; (i)
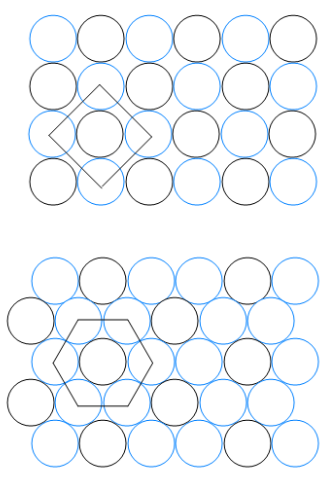
(ii)
Problem 22c
Two patterns of packing two different circles of the same size are shown here. For each structure (c) determine the type of two-dimensional lattice (from Figure 12.4). (i)

(ii)
Problem 23
Imagine the primitive cubic lattice. Now imagine grabbing the top of it and stretching it straight up. All angles remain 90. What kind of primitive lattice have you made?

Problem 25
Which of the three-dimensional primitive lattices has a unit cell where none of the internal angles is 90? (a) Orthorhombic, (b) hexagonal, (c) rhombohedral, (d) triclinic, (e) both rhombohedral and triclinic.
- Besides the cubic unit cell, which other unit cell(s) have edge lengths that are all equal to each other? (a) Orthorhombic, (b) hexagonal, (c) rhombohedral, (d) triclinic, (e) both rhombohedral and triclinic.
Problem 26
- What is the minimum number of atoms that could be contained in the unit cell of an element with a body-centered cubic lattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.
Problem 27
