You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
Ch.12 - Solids and Modern Materials
Chapter 12, Problem 19b
(b) Now draw a picture that represents an amorphous solid at the atomic level.
 Verified step by step guidance
Verified step by step guidance1
Understand that an amorphous solid lacks a long-range order, unlike crystalline solids which have a repeating pattern.
Visualize the atoms or molecules in an amorphous solid as being arranged randomly, without a definite pattern or structure.
Imagine the atoms or molecules as being closely packed together, but not in a regular, repeating lattice.
Consider that the arrangement might resemble a disordered pile of spheres, where each sphere represents an atom or molecule.
Think about how this lack of order affects the properties of the material, such as having no distinct melting point.

Verified Solution
Video duration:
45sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Amorphous Solids
Amorphous solids are materials that lack a long-range order or a well-defined crystalline structure. Unlike crystalline solids, where atoms are arranged in a highly ordered pattern, amorphous solids have a random arrangement of atoms. This disordered structure affects their physical properties, such as melting point and mechanical strength, making them distinct from their crystalline counterparts.
Recommended video:
Guided course
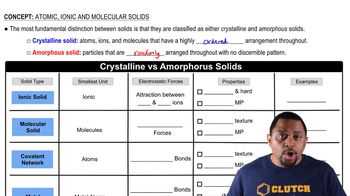
Crystalline vs Amorphous Solids
Atomic Structure
The atomic structure refers to the arrangement of atoms within a material. In the context of amorphous solids, this structure is characterized by a lack of periodicity, meaning that the positions of atoms do not repeat in a regular pattern. Understanding atomic structure is crucial for visualizing how atoms interact and bond in different types of solids, influencing their overall properties.
Recommended video:
Guided course

Atom Structure
Visual Representation of Solids
Visual representation of solids involves creating diagrams or models that depict the arrangement of atoms in a material. For amorphous solids, this representation typically shows a chaotic and irregular distribution of atoms, contrasting with the orderly lattice seen in crystalline solids. Such visualizations help in understanding the unique properties and behaviors of different solid materials.
Recommended video:
Guided course

Crystalline Solids Structure
Related Practice
Textbook Question
500
views
Open Question
You are given a white substance that melts at 100 °C. The substance is soluble in water. Neither the solid nor the solution is a conductor of electricity. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
Textbook Question
(a) Draw a picture that represents a crystalline solid at the atomic level.
927
views
Textbook Question
Two patterns of packing for two different circles of the same size are shown here. For each structure (b) determine the angle between the lattice vectors, g, and determine whether the lattice vectors are of the same length or of different lengths; (i)
(ii)
444
views
Textbook Question
Two patterns of packing two different circles of the same size are shown here. For each structure (c) determine the type of two-dimensional lattice (from Figure 12.4). (i)
(ii)
407
views
Textbook Question
Imagine the primitive cubic lattice. Now imagine grabbing the top of it and stretching it straight up. All angles remain 90. What kind of primitive lattice have you made?
423
views
