Which type (or types) of crystalline solid is characterized by each of the following? (c) high melting point and poor electrical conductivity;
Ch.12 - Solids and Modern Materials
Chapter 12, Problem 17
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Types of Solids
Solids can be classified into four main types: molecular, metallic, covalent-network, and ionic. Each type has distinct properties based on the nature of the bonding and structure. Molecular solids consist of molecules held together by intermolecular forces, metallic solids consist of metal atoms sharing electrons, covalent-network solids have atoms connected by covalent bonds in a continuous network, and ionic solids are composed of ions held together by electrostatic forces.
Recommended video:
Guided course
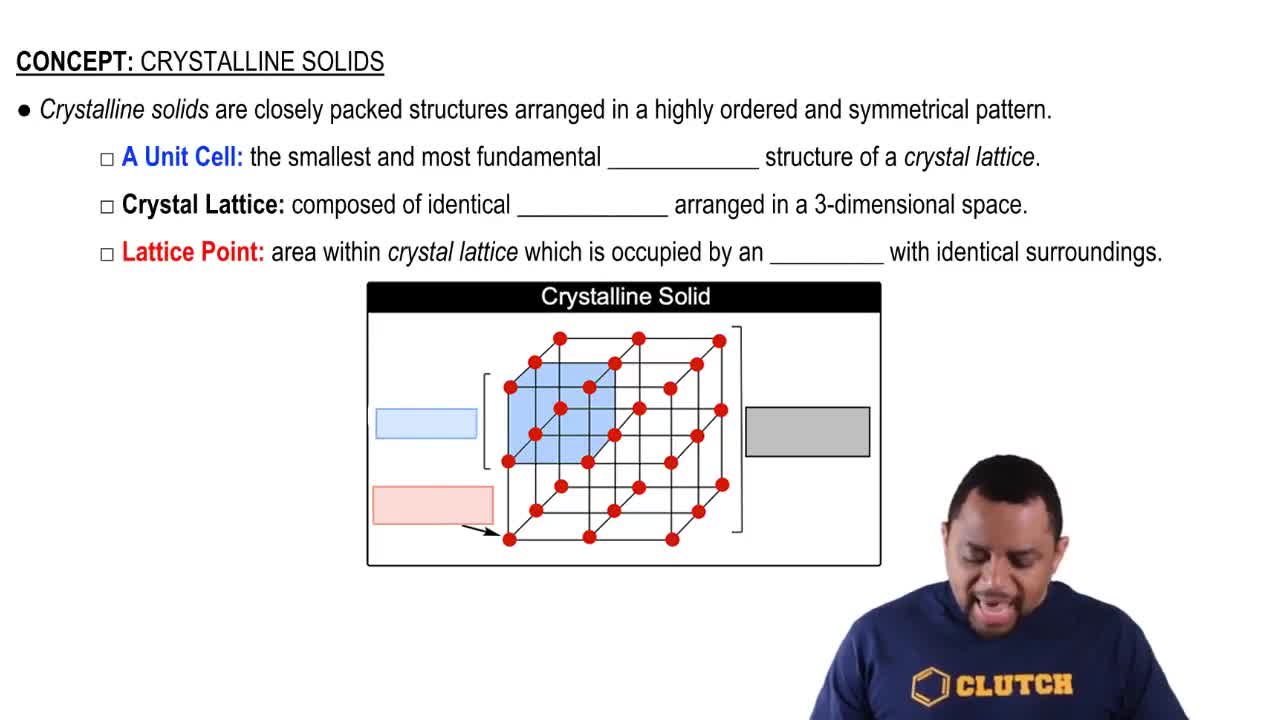
Crystalline Solids Structure
Electrical Conductivity
Electrical conductivity refers to a material's ability to conduct electric current. In solids, this property is influenced by the presence of free-moving charged particles. Metallic solids are good conductors due to the mobility of delocalized electrons, while ionic solids conduct electricity when melted or dissolved in water, as ions can move freely. The gray substance in the question is a conductor, suggesting it may be metallic.
Recommended video:
Guided course
Extensive Property Example
Melting Point and Solubility
The melting point of a substance provides insight into its bonding and structure. A high melting point, like 700 °C, typically indicates strong bonding forces, such as those found in metallic or covalent-network solids. Additionally, solubility in water can help differentiate between types; for instance, ionic solids are often soluble, while metallic and covalent-network solids are generally insoluble. The gray substance's high melting point and insolubility suggest it is likely a metallic solid.
Recommended video:
Guided course
Boiling Point and Melting Point
Related Practice
Textbook Question
483
views
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.
710
views
Textbook Question
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
1258
views
Textbook Question
(a) Draw a picture that represents a crystalline solid at the atomic level.
927
views
Textbook Question
(b) Now draw a picture that represents an amorphous solid at the atomic level.
685
views
Textbook Question
Two patterns of packing for two different circles of the same size are shown here. For each structure (b) determine the angle between the lattice vectors, g, and determine whether the lattice vectors are of the same length or of different lengths; (i)
(ii)
444
views
