Which type (or types) of crystalline solid is characterized by each of the following? (a) High mobility of electrons throughout the solid;
Ch.12 - Solids and Modern Materials
Chapter 12, Problem 14d
Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.
 Verified step by step guidance
Verified step by step guidance1
Identify the key characteristic of the crystalline solid mentioned in the problem, which is a 'network of covalent bonds'.
Understand that a network of covalent bonds in a solid implies that the atoms are bonded in a continuous network across the entire structure, rather than being isolated molecules or ions.
Recall the four main types of crystalline solids: molecular, ionic, metallic, and covalent network.
Analyze which type of crystalline solid typically features a continuous network of covalent bonds. This type of solid is characterized by strong, directional covalent bonds that extend throughout the material, forming a rigid structure.
Conclude that the type of crystalline solid characterized by a network of covalent bonds is known as a 'covalent network solid'.

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Network Solids
Covalent network solids are a type of crystalline solid where atoms are connected by a continuous network of covalent bonds. This structure results in materials that are typically very hard, have high melting points, and are poor conductors of electricity. Examples include diamond and silicon carbide, which exhibit exceptional strength and stability due to their extensive bonding.
Recommended video:
Guided course
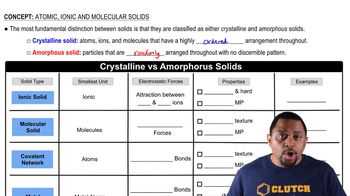
Crystalline vs Amorphous Solids
Crystalline Solids
Crystalline solids are materials whose constituents, such as atoms or molecules, are arranged in an orderly repeating pattern extending in all three spatial dimensions. This regular arrangement leads to distinct geometric shapes and well-defined melting points. Crystalline solids can be classified into various types, including ionic, covalent, metallic, and molecular solids, each with unique properties.
Recommended video:
Guided course

Crystalline Solids Structure
Bonding Types in Solids
The type of bonding in solids significantly influences their physical properties. In covalent network solids, strong covalent bonds create a rigid structure, while ionic solids are held together by electrostatic forces between charged ions. Understanding these bonding types helps predict the behavior of materials under different conditions, such as temperature and pressure, and their suitability for various applications.
Recommended video:
Guided course
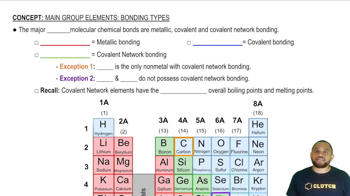
Bonding Types
Related Practice
Textbook Question
1063
views
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (b) softness, relatively low melting point;
781
views
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (c) high melting point and poor electrical conductivity;
483
views
Textbook Question
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
1258
views
Open Question
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (a) InAs, (b) MgO, (c) HgS, (d) In, (e) HBr.
Textbook Question
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
500
views
