Textbook Question
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (a) molecular crystals?
1166
views
 Verified step by step guidance
Verified step by step guidance

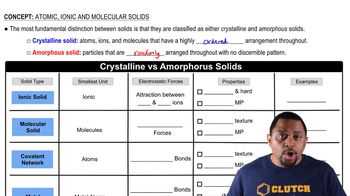

What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (a) molecular crystals?
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (d) and metallic crystals?
Which type (or types) of crystalline solid is characterized by each of the following? (a) High mobility of electrons throughout the solid;
Which type (or types) of crystalline solid is characterized by each of the following? (c) high melting point and poor electrical conductivity;
Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)