What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (a) molecular crystals?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Intermolecular Forces

Molecular Crystals
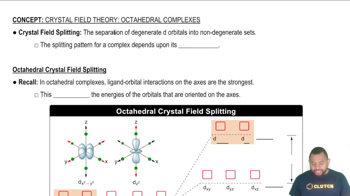
Types of Attractive Forces

Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (a) Is Si a molecular, metallic, ionic, or covalent-network solid?
Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (b) Silicon readily reacts to form silicon dioxide, SiO2, which is quite hard and is insoluble in water. Is SiO2 most likely a molecular, metallic, ionic, or covalent-network solid?
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (d) and metallic crystals?
Which type (or types) of crystalline solid is characterized by each of the following? (a) High mobility of electrons throughout the solid;
Which type (or types) of crystalline solid is characterized by each of the following? (b) softness, relatively low melting point;
