Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.
(a) Draw a picture that represents a crystalline solid at the atomic level.

Verified Solution
Key Concepts
Crystalline Structure
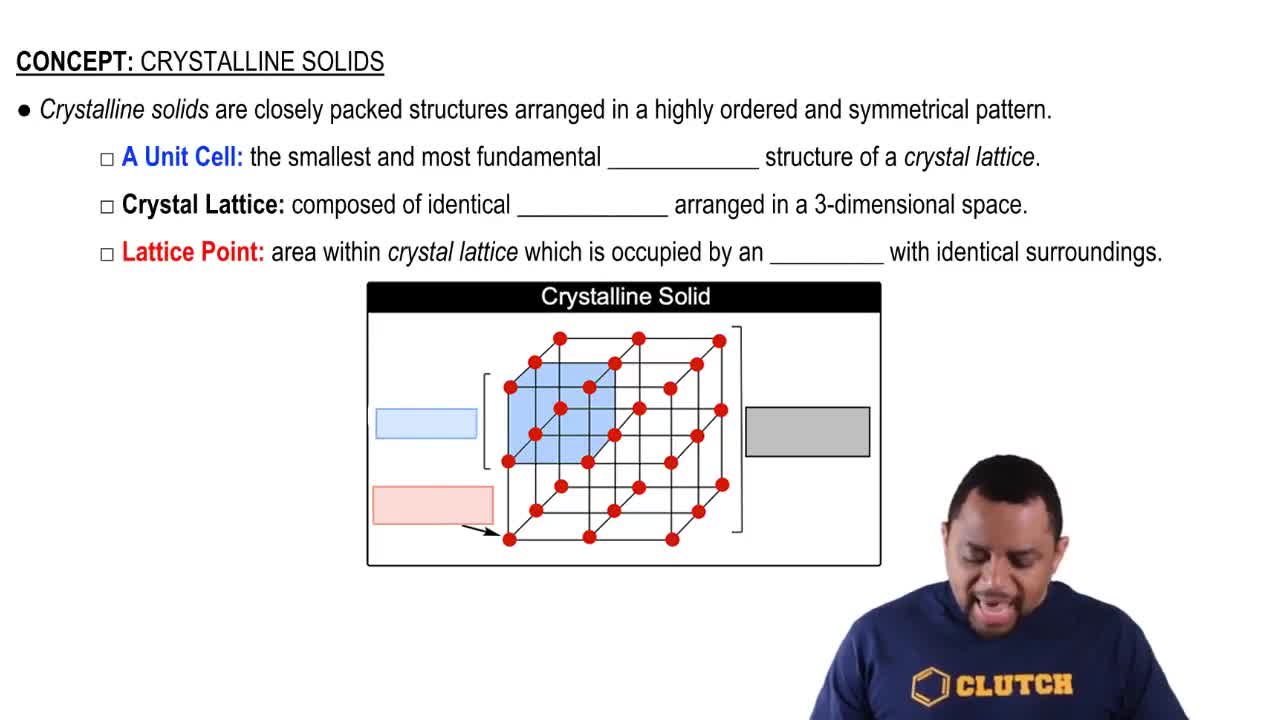
Unit Cell
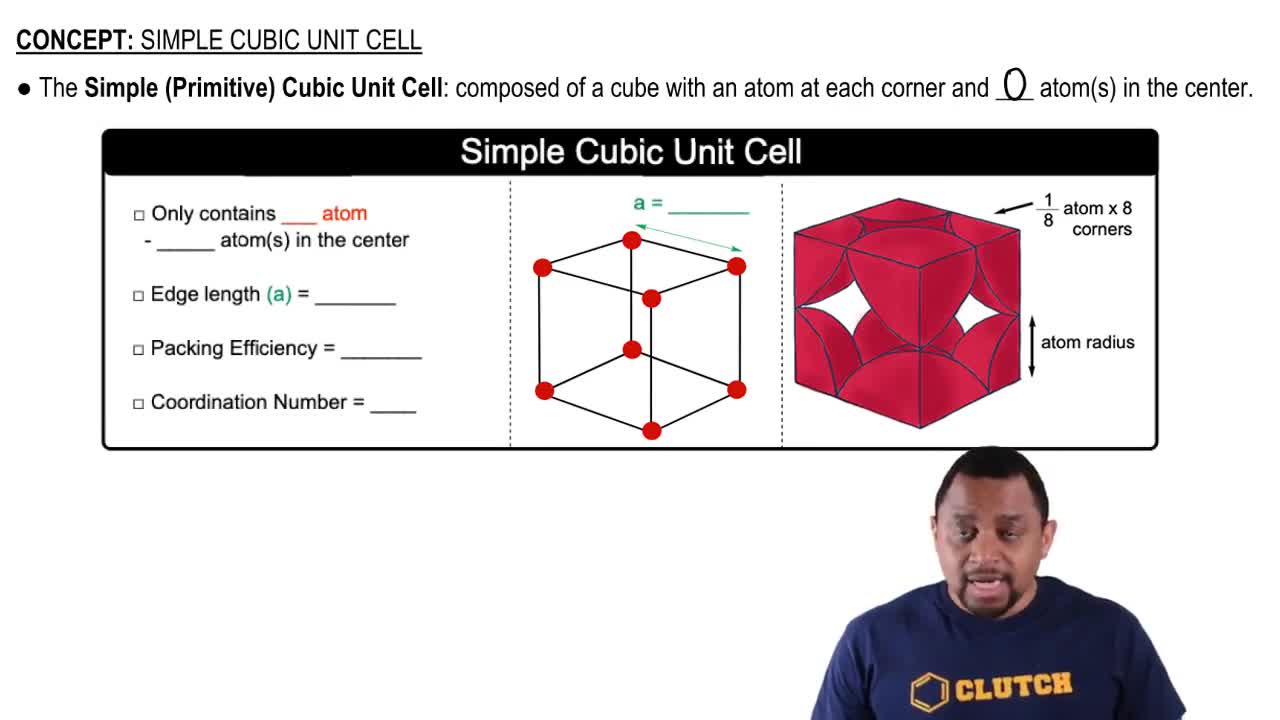
Atomic Bonding

Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
(b) Now draw a picture that represents an amorphous solid at the atomic level.
Two patterns of packing for two different circles of the same size are shown here. For each structure (b) determine the angle between the lattice vectors, g, and determine whether the lattice vectors are of the same length or of different lengths; (i)
(ii)
Two patterns of packing two different circles of the same size are shown here. For each structure (c) determine the type of two-dimensional lattice (from Figure 12.4). (i)
(ii)
