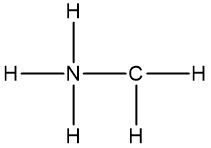
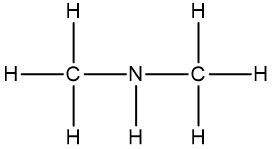
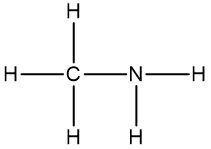
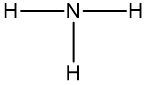
Now molecular formulas are insufficient for organic compounds because they do not convey structural information. Structural information itself gives us an idea about the connectivity of an organic compound, as well as the orientation elements take when they bond to each other within that organic compound. Connectivity is how atoms are connected to each other. Orientation will be discussed later when we talk about stereoisomers. But for right now, let's not worry about it.
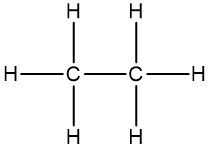
Molecular representation: Here we have our molecular formula of C2H6O. This structure can be drawn in a couple of ways. One potential way of drawing this is having our two carbons connected to each other, and then we're going to assign hydrogens to these carbons. Remember, carbon ideally wants to make four bonds. So far, we've used both carbons and five hydrogens. What we have left is an oxygen and a hydrogen. We could then write an oxygen connected to this carbon, and then place the remaining hydrogen on it. So, this is one potential structure from the molecular formula.
Now, there's just not enough information, just from this molecular formula because we could also draw it as our two carbons, each connected to the oxygen, and each carbon having three hydrogens. Technically, either one of these structures is correct if we're only given the molecular formula. As you can see, we don't have enough information to know for sure which one of these two structures the person might want. Right? So, that's the limitation when it comes to molecular formulas. We might know the number of elements, but we don't know how they are connected to one another, and what their orientation would be in terms of space.
Just remember, this is one of the limitations when it comes to molecular formulas.