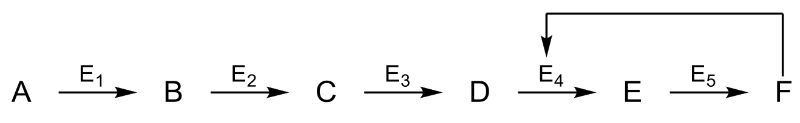
A metabolic pathway consists of a series of biochemical reactions occurring within a cell, where feedback control plays a crucial role in regulating enzyme activity. In this context, the end product of a pathway functions as a negative allosteric regulator, effectively acting as an on-off switch for the enzyme involved in the initial step of the pathway.
To understand this process, consider an enzyme with an active site where a substrate, denoted as A, can bind. The enzyme also contains an allosteric site, which is currently unoccupied. When the substrate binds to the active site, an enzyme-substrate complex is formed, facilitating the conversion of the substrate into intermediates, ultimately leading to the production of the final product, denoted as D.
Once a sufficient quantity of product D is synthesized, it binds to the allosteric site of the enzyme. This binding alters the enzyme's conformation, rendering the active site unable to accommodate the substrate. Consequently, the enzyme becomes inactive, preventing further production of product D. This mechanism is essential for maintaining homeostasis within the cell, as it prevents the overproduction of metabolites.
As the concentration of product D decreases, it may detach from the allosteric site, allowing the enzyme to regain its active form. This cycle of activation and deactivation exemplifies feedback control in enzyme regulation, ensuring that metabolic pathways operate efficiently and responsively to the cell's needs.