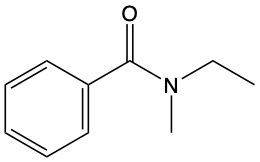
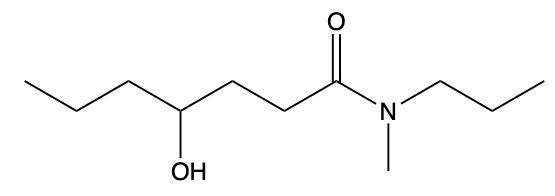
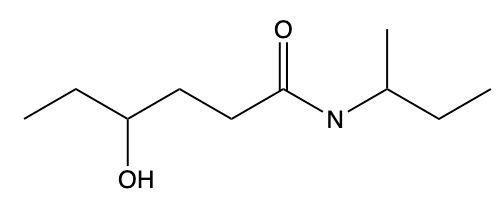
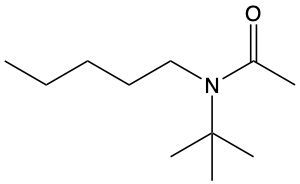
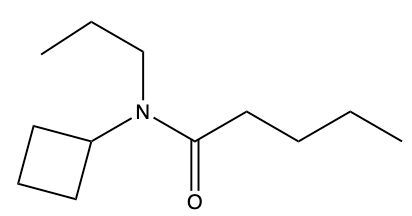
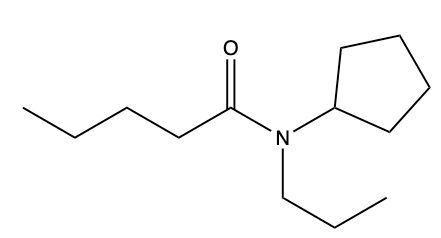
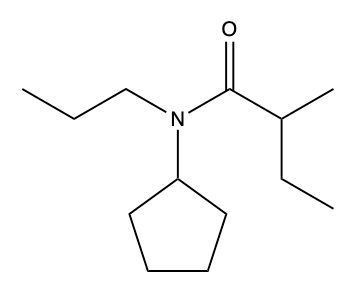
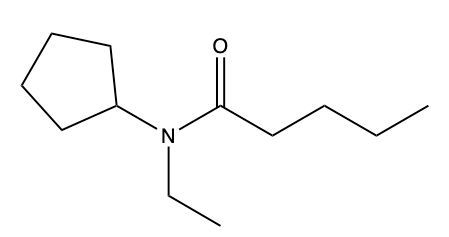
Amides are a class of organic compounds characterized by the presence of a carbonyl group (C=O) directly connected to a nitrogen atom (N). This nitrogen atom typically originates from an amine group. Amides can be classified into three categories based on the number of carbon atoms directly attached to the nitrogen: primary, secondary, and tertiary amides.
A primary amide has one carbon atom attached to the nitrogen. For example, in a primary amide structure, the nitrogen is bonded to one carbon and two hydrogen atoms. In contrast, a secondary amide features two carbon atoms connected to the nitrogen, which means the nitrogen is bonded to one carbon and one other carbon atom. Lastly, a tertiary amide contains three carbon atoms directly attached to the nitrogen, indicating that the nitrogen is bonded to three carbon atoms and no hydrogen atoms.
This classification is essential for understanding the reactivity and properties of amides, as the number of substituents around the nitrogen influences their chemical behavior. Recognizing these distinctions allows for better comprehension of amide structures and their applications in various chemical reactions.