Oftentimes, when dealing with calculations, we'll run into contact with the metric prefixes. Now, metric prefixes can be seen as modifiers that are multiples of 10. And we're going to say when dealing with these metric prefixes, we're going to have this chart here. This metric prefix multipliers chart ranges from 1012 to 10-12. Now talk to your professor. Make sure you don't have to know anything beyond this point. For this level of chemistry, this is a pretty thorough range to deal with. But there, of course, there are terms 10-15, 1015. Well, those are usually reserved for higher levels of chemistry. Now 1012 is called terra. Terra uses the variable of t, capital T. 109 is giga, which is capital G. Mega is capital M, and it's 106. 103 is kilo. Now, at this point we're gonna be dealing with lowercase letters, so lowercase k. You might have heard of kilometer. So kilometer has kilo in it. It's metric prefix label. 102 is hecto, which is h. Now 101 is related to deka, which is da. Here is when we're dealing with our base unit. So our base unit like liters or seconds. We're gonna say here this is not a metric prefix, this is just the base, our base unit. Then we have deci. Dep and deci are pretty similar. To differentiate them, deci is d. Then we're gonna have centi, which is 10-2. So that's gonna be c. You might have heard of centimeters. Then we have 10-3 which is milli, milliliters. Then 10-6 is micro. Micro is an interesting symbol. 10-9 is nano, which is lowercase n, and then 10-12 is pico, which is p. Now this is a lot of terms. This is a lot of symbols, but we have our first memory tool. So when we have memory tools, either just simple phrases sometimes or images that'll help us memorize a specific chemistry-related topic. Here in this case, this memory tool helps us memorize the order for the metric prefix multipliers. Now we have King Henry from history, King Henry who kept on divorcing his wives until they wanted them to compare him a son. And with King Henry, we have a trusty memory tool to help us memorize the order of the metric prefixes. So we're gonna say the great monarch, King Henry's daughter Barbara, drinks chocolate milk until 9 pm. So you can see here that each one of these highlighted letters, each one of these highlighted letters here, corresponds to these metric prefix multipliers. And this memory tool also ranges from 1012 and it decreases all the way down to 10-12 over here. So just remember, your metric prefixes for us, we're gonna range from 1012 to 10-12. Consult with your professor to make sure that that's the range all you need to know in terms of this topic. And in terms of memorizing, rely on this memory tool. It'll help you remember the order that each metric prefix multiplier comes into play when it comes to this chart. Now that we've talked about the general idea of metric prefixes and this range, move on to the next video, and let's see how we can apply them to base units.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Metric Prefixes - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIMetric prefixes are essential modifiers in chemistry, representing powers of ten. Common prefixes include tera (1012), giga (109), mega (106), kilo (103), and milli (10-3). These prefixes can be applied to base units like liters, seconds, moles, and amperes, facilitating conversions such as milliliters or nanoseconds. A mnemonic, "King Henry's Daughter Barbara Drinks Chocolate Milk Until 9 PM," aids in memorizing the order of prefixes. Understanding these prefixes is crucial for accurate measurements and calculations in scientific contexts.
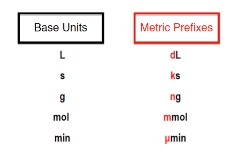
Metric Prefixes are “labels” that can be placed in front of base units.
Metric Prefix Multipliers
Metric Prefixes
Video transcript
Metric Prefixes
Video transcript
The metric prefixes themselves can act as labels that can be placed in front of various base units. So here we have base units for volume in the form of liters, in terms of time we use seconds, the base units for the amount of a substance is moles, and for electrical current amperes. Notice that some of these common base units are also connected to our SI base units. Now, the metric prefixes themselves are the variables that we place in front of each of these base units. So for liters, we could use milliliters, with milli being the metric prefix that we're placing in front of the base unit of liters. For seconds, we could use nanoseconds. For moles, we could use gigamoles, and for amperes, we could use kiloamperes. Here, all that's happening is I'm taking each one of these metric prefixes and placing them in front of one of these base units. This is key when it comes to metric prefix conversions. So click on the next video, and let's take a look at how we go from one metric prefix to a new one.
Metric Prefixes Example 1
Video transcript
So here if we take a look at this example, it says, convert the following value to desired units. We need to convert 694 kilograms to micrograms. Alright. So step 1, if the given value has a metric prefix, which it does because 'kilo' is our metric prefix, then convert it to the base unit. The base unit is just a unit after you've removed the metric prefix. So the base unit here would just be grams. Alright. So we're gonna have 694 kilo is our metric prefix, grams. We're gonna say in order to cancel out units, always make sure that they are on opposite levels. So, we want to cancel out these kilograms which are on the top, so we're gonna place kilograms here on the bottom. And then we need to change this into our base units of grams. Now the next part is important. Always place the coefficient of 1 on the side with the metric prefix. Our metric prefix here is 'kilo', so we're gonna put here 1 kilo, and remember that comes from what we saw up above. Based on the metric prefix multipliers chart, we saw that when it is 'kilo', it's 103, so it'd be 103. Now because the kilograms are on opposite levels, they can cancel out. Next, we're gonna say, if necessary, convert the base unit to a new metric prefix. Now we didn't ask to find our answer in grams. We're asked to find it in micrograms. So we must continue onward. Grams are here on the top. In order to cancel them out, I put them here on the bottom, And then we need to get to micrograms, micro is our metric prefix, and then micrograms. Again, the coefficient of 1 is associated with the metric prefix, it's on the same side with it. So 1 micro is, according to the metric prefix multipliers chart above, it's 10-6. So now grams cancel out and we'll have at the end our value. So here that would be 694 times 103 divided by 10-6. Now, some of you, depending on your calculator, if you plug it in as you see it, you might get the wrong answer. So anytime you have 103, it's always best to put it in parentheses in your calculator. Otherwise, you may get the incorrect answer. So it'd be 694 times, in parentheses, 103, divided by, in parentheses, 10-6. If you do that correctly, you should get back in your calculator 6.9411 micrograms as your answer. And this represents our metric prefix conversions. We're just going from a metric prefix to either the base unit or a new metric prefix. Take to heart the steps that we've outlined here to approach any problem like this. Now that we've done this example question, move on to some practice questions.
Which quantity in the following pair is smaller?
Use the prefix multipliers to express each measurement without any exponents.
a) 32 x 10-13 L
b) 7.3 x 106 g
c) 18.5 x 1011 s
Problem Transcript
Use scientific notation to express each quantity with only the base unit.
a) 83 µm
b) 193 kg
c) 2.7 mmol
Problem Transcript
If a room has a volume of 1.15 x 108 cm3, what is the volume in km3?
Do you want more practice?
Here’s what students ask on this topic:
What are the common metric prefixes and their corresponding powers of ten?
Common metric prefixes and their corresponding powers of ten include:
- Tera (T): 1012
- Giga (G): 109
- Mega (M): 106
- Kilo (k): 103
- Hecto (h): 102
- Deka (da): 101
- Base unit: 100
- Deci (d): 10-1
- Centi (c): 10-2
- Milli (m): 10-3
- Micro (μ): 10-6
- Nano (n): 10-9
- Pico (p): 10-12
These prefixes are used to modify base units like liters, seconds, moles, and amperes, making it easier to express very large or very small quantities.
 Created using AI
Created using AIHow can I memorize the order of metric prefixes?
A helpful mnemonic to memorize the order of metric prefixes is: "King Henry's Daughter Barbara Drinks Chocolate Milk Until 9 PM." Each word's initial corresponds to a prefix:
- King (Kilo, 103)
- Henry (Hecto, 102)
- Daughter (Deka, 101)
- Barbara (Base unit, 100)
- Drinks (Deci, 10-1)
- Chocolate (Centi, 10-2)
- Milk (Milli, 10-3)
- Until (Micro, 10-6)
- 9 (Nano, 10-9)
- PM (Pico, 10-12)
This mnemonic helps in recalling the order from larger to smaller prefixes.
 Created using AI
Created using AIHow do metric prefixes facilitate conversions in scientific measurements?
Metric prefixes facilitate conversions by providing a standardized way to express large and small quantities. For example, instead of writing 0.000001 liters, you can write 1 microliter (μL). This simplifies calculations and communication in scientific contexts. By using prefixes like milli (10-3), micro (10-6), and kilo (103), you can easily convert between units. For instance, 1 kilometer (km) is 1000 meters (m), and 1 milliliter (mL) is 0.001 liters (L). Understanding these prefixes is crucial for accurate measurements and conversions in chemistry, physics, and other sciences.
 Created using AI
Created using AIWhat are some examples of metric prefixes applied to base units?
Metric prefixes can be applied to various base units to express different magnitudes. Here are some examples:
- Volume: Milliliters (mL) - 10-3 liters
- Time: Nanoseconds (ns) - 10-9 seconds
- Amount of substance: Gigamoles (Gmol) - 109 moles
- Electrical current: Kiloamperes (kA) - 103 amperes
These prefixes help in expressing very large or very small quantities in a more manageable form, making calculations and communication more efficient in scientific work.
 Created using AI
Created using AIWhy are metric prefixes important in scientific measurements?
Metric prefixes are important in scientific measurements because they provide a standardized way to express and convert large and small quantities. This standardization ensures consistency and accuracy in scientific communication and calculations. For example, using prefixes like kilo (103), milli (10-3), and micro (10-6) allows scientists to easily convert between units and understand the scale of measurements. This is crucial in fields like chemistry, physics, and engineering, where precise measurements are essential for experiments, data analysis, and technological development.
 Created using AI
Created using AIYour GOB Chemistry tutor
- Write the abbreviation for each of the following units: c. kilometer
- Write the abbreviation for each of the following units: b. deciliter
- Write the abbreviation for each of the following units: a. milligram
- Write the complete name for each of the following units: a. cL
- Write the complete name for each of the following units: b. kg
- Write the complete name for each of the following units: c. ms
- Write the numerical value for each of the following prefixes: c. milli
- Write the numerical value for each of the following prefixes: b. tera
- Write the numerical value for each of the following prefixes: a. centi
- Use a prefix to write the name for each of the following: b. 10⁶ m
- Use a prefix to write the name for each of the following: c. 0.001 m
- Use a prefix to write the name for each of the following: d. 10⁻¹² m
- For each of the following pairs, which is the larger unit? c. m or km
- For each of the following pairs, which is the larger unit? b. milliliter or microliter
- For each of the following pairs, which is the larger unit? a. milligram or kilogram
- Give the full name of the following units: a. cc b. dm c. mm d. nL e. mg f. m³
- Use metric conversion factors to solve each of the following problems: d. A balloon has a volume of 3500 cm³....
- Use metric conversion factors to solve each of the following problems: c. A hummingbird has a mass of 0.0055 ...
- Use metric conversion factors to solve each of the following problems: b. A cooler has a volume of 5000 mL. W...
- Use metric conversion factors to solve each of the following problems: d. A jar contains 0.29 kg of olives. H...
- Use metric conversion factors to solve each of the following problems: c. A package of chocolate instant pudd...
- Use metric conversion factors to solve each of the following problems: a. The Daily Value (DV) for phosphorus...
- Use metric conversion factors to solve each of the following problems: b. A glass of orange juice contains 3....
