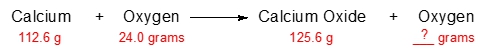In 1789, the French chemist, Antoine Lavoisier, who's credited as being another father of chemistry, originated the law of conservation of mass. Now, the law states that in a chemical reaction, no matter is created or destroyed, but instead changes form.
In a chemical reaction, we're going to say that the compounds before the arrow, so in this case H2+O2, would be our reactants, and those after the arrow, in this case, H2O, would be our products. According to Lavoisier, all other reactants are converted to product with nothing lost. So to think about it, we could say that originally at the beginning of the reaction, we have H2 and O2, and we're mixing them together. Their combined amount of both of them together, let's say it's 100 grams. Well, according to the law of conservation of mass, nothing is truly lost. It's just converted from one form to another.
So all 100 grams of my reactants should be converted entirely into product. So at the end, I'd have exactly 100 grams of product. All of this would be gone. All of this has been transformed into H2O. So what this tells me is that if you know the total mass of your reactants in the beginning, then they should equal the total mass of products that you create at the end. So this is an incredibly important idea when it comes to the law of conservation of mass. That'll connect to other ideas later on such as stoichiometry and solution chemistry. So just remember, when it comes to law of conservation of mass, it helps us to determine eventually the amount of product we could potentially form.