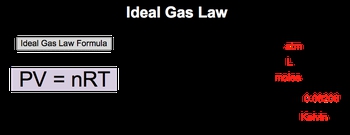
In our exploration to describe the behavior of gases, we come upon the ideal gas law. Now the ideal gas law formula relates the behavior of gas under varied conditions of pressure, volume, moles, and temperature. So our ideal gas law formula is PV=nRT. We see that it's in a purple box, which means that you need to memorize it. Your professor is going to expect you to know this formula. It's going to be involved in so many different types of ideas and calculations within this chapter. Now if we take a closer look at it, we said that P equals pressure. Here, the units for pressure will be in atmospheres. Volume will be in liters, n equals the amount of gas in moles. R is a gas constant, and here when it comes to the ideal gas law, we tend to see it as 0.08206. In the next section, we'll talk about the units involved with this gas constant. And then temperature here, temperature will be in Kelvins. So just remember, to talk about the ideal behavior of gases, we use the ideal gas law. So click on the next video, and let's take a look at an example question.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
The Ideal Gas Law - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIThe ideal gas law, represented by the equation , describes the behavior of gases in terms of pressure (P), volume (V), moles (n), and temperature (T). The gas constant (R) can be 0.08206 L·atm/(mol·K) for the ideal gas law or 8.314 J/(mol·K) for energy-related calculations. Understanding these constants and their applications is crucial for solving gas-related problems in chemistry.
The Ideal Gas Law formula relates the behavior of gases under varying conditions of pressure, volume, moles and temperature.
The Ideal Gas Law
The Ideal Gas Law
Video transcript
The Ideal Gas Law Example 1
Video transcript
Here we're told that a 500 milliliter container at a pressure of 600 milliliters of mercury possesses 29.3 grams of nitrogen gas at 50 degrees Celsius. Here we need to determine the correct units needed for the ideal gas law. So remember, the ideal gas law is PV=nRT. So P stands for pressure. Remember pressure needs to be in units of atmospheres. So here, we're going to do a conversion. We're going to go from 600 millimeters of mercury to atmospheres. Millimeters of mercury go here on the bottom, and then atmosphere goes on top. Remember the relationship is that for every one atmosphere, it's 760 millimeters of mercury. So when we do that, we're going to get a pressure of 0.789 atmospheres. Although 600 has one significant figure, let's just go with three significant figures here, just so we don't have just 0.8 as atmospheres.
Next, volume. Volume needs to be in liters, so we have 500 milliliters. And remember, 1 milli equals \(10^{-3}\). So this would just come out as 0.500 liters.
Next, we need moles, so moles is n. Remember nitrogen gas. Nitrogen in its natural or standard state is N2. It's one of our diatomic molecules. So we're told that we have 29.3 grams of N2. We must convert that into moles, so 1 mole of N2. There are 2 nitrogens. According to the periodic table, each is 14.01 grams. So multiplying that by 2 gives me 28.2 grams for the combined weight in N2. So grams here cancel out and I'll have my moles. Here we'll put it as 1.05 moles of N2.
And then finally, we need our temperature. R is our constant, so we don't have to worry about providing the correct units. It's 0.08206 like we said earlier. So temperature here needs to be in Kelvin. So remember to go from Celsius to Kelvin, you're supposed to add 273.15. Sometimes professors will just say plus 273, but if you want to be as accurate as possible, you want to put 273.15. So when you add that, that's 323.15 Kelvin. So here we have all of the units that we need in the correct forms. So just remember the ideal gas law and what are the correct units that we need to utilize for each one of these variables.
The Ideal Gas Law
Video transcript
As we said, the ideal gas law uses r, which is our gas constant, but realize that the gas constant r can have 2 different values depending on the situation. Now the 2 terms you'll tend to see when it comes to the r constant is the first one we talked about earlier, r being equal to 0.08206. We utilize this value when dealing with the ideal gas law, but r can also equal 8.314. This happens when we're dealing with speed, velocity, or energy. So if a question is dealing with the speed of an object, or a subatomic particle, or dealing with the energy of a reaction or solution, the r converts to 8.314. Now the conversion factor between the r groups is that 1 liter times atmospheres equals 101.325 joules. So we have our 0.08206 here. We use the conversion factor. So remember, we want to get rid of liters times atmospheres, so we put them on the bottom. So 1 liter times atmosphere here on the bottom is 101.325 joules here on top. Liters times atmospheres cancel out and that's how we end up with joules at the end. When you plug this in, you get a long string of numbers as r8.3147295J/mol∙K joules over moles times K. But we just focus on the 8.314 portion. I know there's a 7 there when you work it out completely, but to keep it simple, books will just list it as 8.314. So just keep in mind the gas constant can be 2 different numbers. And remember with the ideal gas law, we use this value, and when dealing with speed, velocity, or energy, we use this value of 8.314.

The Ideal Gas Law Example 2
Video transcript
Here in this example question, it says, how many moles of ammonia are contained in a 25-liter tank at 190 degrees Celsius and 5.20 atmospheres. Alright. So they're asking me to find moles of ammonia. So that would be my n variable. They're giving me liters, so that would be volume. They're giving me degrees Celsius which is temperature, and they're giving me atmospheres which is a unit for pressure. We can see that we have PV = nRT, so we have the ideal gas law to deal with. And the variable that's missing, the one that we're looking for is moles. So all we have to do here is isolate moles. So divide both sides here by RT, and then we're going to say here that moles equals PV over RT. Pressure is already in atmospheres, so no need to convert. Then we're going to have here volume which is already in liters so no need to convert. Then we have our R constant, remember, is 0.08206. And now that we know that we're using the ideal gas law, we have to use the units of liters times atmospheres per moles times Kelvin.
Temperature, remember, has to be in Kelvin. So there is a little bit of converting here necessary. So we have 190 degrees Celsius, so add 273.15 to that and that'll give us 463.15 Kelvin. So take those and plug it in, 463.15 Kelvin. So what cancels out? Atmospheres cancel out, liters cancel out, Kelvins cancel out, and you'll see that the only unit left are the moles that we need to find. So we're going to get initially 3.4205 moles. If we look back on the question, this has 3 significant figures, 2 significant figures, and 3 significant figures. We go with the least number of significant figures, so we're going to put our answer as 3.4 moles of ammonia. So this would be our final answer. So just realize what variables you are being given, what's the missing variable, just use your algebra skills to isolate that one variable, and you'll get your answer at the end.
How many grams of carbon dioxide, CO2, are present in a 0.150 L flask recorded at 525 mmHg and 32 ºC?
1.77 g
0.93 g
0.66 g
0.18 g
0.052 g
How many liters of HNO3 gas, measured at 28.0 ºC and 780 torr, are required to prepare 2.30 L of 4.15 M solution of nitric acid?
When 0.670 g argon is added to a 500 cm3 container with a sample of oxygen gas, the total pressure of the gases is found to be 1.52 atm at a temperature of 340 K. What is the mass of the oxygen gas in the bulb?
Do you want more practice?
Here’s what students ask on this topic:
What is the ideal gas law and its formula?
The ideal gas law is a fundamental equation in chemistry that describes the behavior of gases under various conditions of pressure, volume, moles, and temperature. The formula for the ideal gas law is given by:
where is the pressure in atmospheres, is the volume in liters, is the number of moles of gas, is the gas constant, and is the temperature in Kelvins. The gas constant typically has a value of 0.08206 L·atm/(mol·K) for the ideal gas law.
 Created using AI
Created using AIWhat are the units for the gas constant R in the ideal gas law?
In the context of the ideal gas law, the gas constant has the value 0.08206 L·atm/(mol·K). This means that the units for are liters times atmospheres per mole per Kelvin. These units ensure that when you multiply by the number of moles and temperature, and divide by volume, you get the pressure in atmospheres.
 Created using AI
Created using AIWhen do you use the gas constant value 8.314 J/(mol·K)?
The gas constant value 8.314 J/(mol·K) is used in situations involving energy, speed, or velocity. For example, when dealing with the kinetic energy of gas particles or the energy changes in a chemical reaction, you would use this value of . The conversion factor between the two values of is that 1 L·atm equals 101.325 J.
 Created using AI
Created using AIHow do you convert between the two values of the gas constant R?
To convert between the two values of the gas constant , you use the conversion factor that 1 L·atm equals 101.325 J. For example, to convert 0.08206 L·atm/(mol·K) to J/(mol·K), you multiply:
This gives you the value 8.314 J/(mol·K), which is used in energy-related calculations.
 Created using AI
Created using AIWhy is it important to use Kelvins for temperature in the ideal gas law?
It is important to use Kelvins for temperature in the ideal gas law because the Kelvin scale is an absolute temperature scale. This means it starts at absolute zero, the point where all molecular motion ceases. Using Kelvins ensures that the temperature is always positive, which is necessary for the calculations in the ideal gas law to be accurate. Converting Celsius to Kelvin is simple: just add 273.15 to the Celsius temperature.
 Created using AI
Created using AI