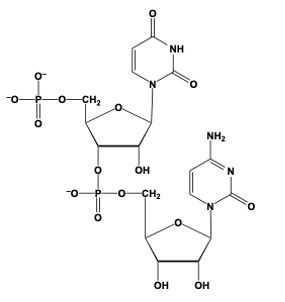
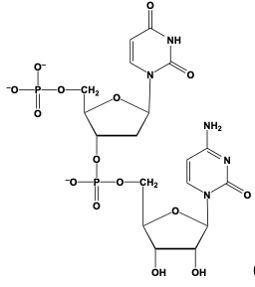
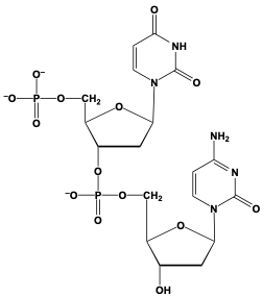
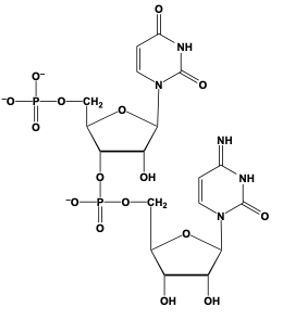
Hey, everyone. So when we say phosphodiester bond formation, we mean that the sugar of a nucleotide plus the phosphate group of another nucleotide equals an ester bond. Now, here we're going to say this is accomplished by the C3' carbon OH group of one nucleotide, so this carbon in question, and it's replacing an oxygen of the phosphate group in another nucleotide. Right. So this oxygen here that I have highlighted, we'll use that one as the one we're replacing, but in actuality it could be any of these oxygens—this one, this one, or this one—of the phosphate group. So, here we'll just say this one.
Now, what's going to happen is we're going to draw this first nucleotide to the left, and here is its pentose ring. Just for simplicity, write the nitrogenous group as just "N" in a box. We still have our C5' carbon still connected to its phosphate group. And then this oxygen in OH, it's going to replace this oxygen in purple that I circled, and it's going to be connected to the rest of this phosphate group here. This phosphate group is still connected to the rest of its nucleotide. So we're going to have here, the CH2 at the C5' prime carbon of this nucleotide still connected to its pentose sugar with its OH on the C3' prime carbon, and its nitrogenous base here.
So here we're talking about our phosphodiester bonds. We're really talking about the connections that we have here in reference to our phosphodiester bond formation. So we basically just linked up two nucleotides for the use of these phosphodiester bonds. Alright. So that's the gist of what happens here in terms of when two nucleotides are reacting, and we have to connect them together. It's a relation to the C3' carbon OH of one nucleotide interacting with the phosphate group of another nucleotide.