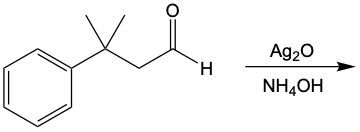
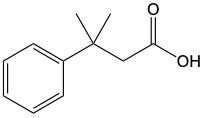
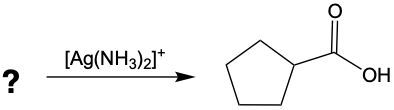
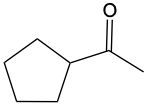
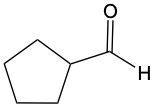
Now, when it comes to Tolan's test and Benedict's test, they basically function as oxidation reactions. Recall that oxidation uses an oxidizing agent to add as many carbon-oxygen bonds as possible without breaking any carbon-carbon bonds. When it comes to oxidations with Tollens' test and Benedict's test, we're paying attention to what's shaded within our box. Looking outside that box, initially we look at alkanes. It is said that alkanes can undergo oxidation to typically make alcohols. Alcohols themselves can be oxidized, where the carbon with the OH group becomes a carbonyl group. In this instance, this alcohol gets oxidized into an aldehyde, and that aldehyde can undergo further oxidation. We're trying to make as many carbon-oxygen bonds as possible. To do that, we're going to have to break one of these carbon-hydrogen bonds. Here, this H is going to be oxidized or removed, and in its place, we're going to have an oxygen placed. Oxygen needs to make two bonds, so it will be accompanied by a hydrogen. In this instance, we've gone from an aldehyde to a carboxylic acid. Now, aldehydes can be oxidized into carboxylic acids. Ketones, because they have a carbon on both sides, would not undergo oxidation. We're not trying to break any carbon-carbon bonds. We wouldn't be able to break this bond or this bond to add an oxygen to that carbonyl carbon. So, here we're just going from an aldehyde to a carboxylic acid. If we wanted to, we could go further on to a carbon dioxide molecule. But again, we are only focusing on this portion where we have an aldehyde being oxidized into a carboxylic acid.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Tollens' and Benedict's Test - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AITollens and Benedict tests are oxidation reactions used to identify aldehydes. Tollens test, known as the silver mirror test, uses silver oxide and ammonia to oxidize aldehydes into carboxylic acids, producing a silver precipitate. Benedict's test similarly oxidizes aldehydes in a basic solution, reducing copper(II) ions to form a brick red copper(I) oxide precipitate. Both tests confirm the presence of aldehydes through distinct visual changes, highlighting the importance of oxidation in organic chemistry.
Oxidation Reactions Concept 1
Video transcript
Tollens' and Benedict's Test Example 1
Video transcript
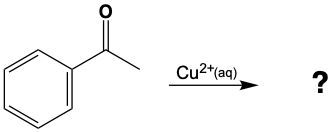
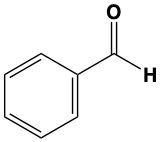
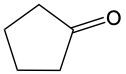
Determine which of the following compounds cannot undergo an oxidation reaction. So, if we take a look at our choices here, we have hexanol, benzaldehyde, acetone, ethanol, and propanol. Now if we take a close look at their names, we see that a, d, and e end with "ol," meaning that they are alcohols. Remember, alcohols can be oxidized to aldehydes or ketones, so they can undergo oxidation. Now b and c, benzaldehyde, that is a benzene ring with an aldehyde attached to it. It's in the name "aldehyde." Since it's an aldehyde, it can also undergo oxidation to a carboxylic acid. The odd one out, the one that cannot undergo oxidation, is acetone. Acetone is a common name for ketone. Remember, acetone is a CH3 group connected to a carbonyl group, connected to another CH3 group. This cannot undergo oxidation because it would require us to cut through carbon-carbon bonds, which we're not allowed to do under typical oxidation reactions. So here, option c would be our correct answer. Acetone cannot undergo an oxidation reaction.
Tollens' Test Concept 2
Video transcript
Now, Tollen's test, it tests for the presence of an aldehyde within a basic solution. Here we're going to say that it's also known as the silver mirror test, because of the formation of a silver precipitate; remember precipitate just means a solid.
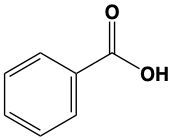
Now, here if we take a look at the reaction for Tollens' test, we have a typical aldehyde here and with it, we're reacting it with silver oxide and we have aqueous ammonia. "Aqueous ammonia" just means we have NH3 dissolved in water. And then we have the addition of heat. This causes my aldehyde to be oxidized into a carboxylic acid. During this process, if we have an aldehyde and it gets oxidized to a carboxylic acid, we'd have the reduction of our silver, which would create a silver solid. So you make a silver solid as a product.
Now in the lab, you're doing this within a test tube and if your unknown happens to be an aldehyde, the reaction will be successful and give a positive result. The positive result will be the depositing of silver at the bottom of your test tube, making it look like a silver mirror. So that's where its name comes from. If you were to do this in a lab and you have a test tube, if you have an aldehyde as your unknown, look at the bottom of the test tube. You'll see silver deposits on the bottom, almost reflective. It's a silver mirror.
Now, here we're going to say the oxidizing agent can be presented in different ways. As a combination of silver, so Ag, and some type of ammonia. So some type of nitrogen-containing compound. Right? This is the way I've depicted it here, but we'll see other combinations that still qualify it as being a Tollen's test. So keep that in mind.
Tollens' Test Example 2
Video transcript
Draw the carboxylic acid produced when 2,3-dimethylpentanal is submerged in a Tollens solution. Alright. So here we know that Tollens' test will change our aldehyde into a carboxylic acid. Let's draw what 2,3-dimethylpentanal would look like. So "pent" means we have 5 carbons, and "al" means we have an aldehyde. So we're going to say that one of the end carbons is an aldehyde carbon. So it's that one. And where it's located is 1. So 1, 2, 3, 4, and 5. Then we have 2,3-dimethyl. Those are our substituents, and they're located on carbons 2 and 3. So on carbon 2, there's a methyl, and in carbon 3, there's a methyl. This is undergoing an oxidation by Tollens' test. So remember, it's a combination of silver with some type of ammonia compound. Here I'm just going to place O to show that it's undergoing oxidation. So our aldehyde group will just get oxidized into a carboxylic acid. So that would look like this. And then on carbons 2 and 3, we still have our methyl groups. So this would be the carboxylic acid formed from the oxidation of our aldehyde by Tollens' test.
Benedict's Test Concept 3
Video transcript
Here we're going to say that, like the Tollens' test, the Benedict's test tests for the presence of an aldehyde within a basic solution. So here we're going to say that a positive Benedict's test reduces copper(II) ion to copper(I) ion in the formation of a brick red copper(I) oxide precipitate. Remember, precipitate just means solid. Also remember that brick red is the exact name of the color you need to remember for a positive Benedict's test. If we take a look here at our equation, we're gonna say under Benedict's test, we have a typical aldehyde. It's going to be reacting with some copper(II) basic solution. As a result of this, the aldehyde gets oxidized into a carboxylic acid. So here's the formation of our carboxylic acid. And then we have copper(I) oxide as a byproduct. That gives us a brick red color to our test tube that would show that we have a positive Benedict's test that are unknown somewhere within it had an aldehyde that got oxidized to a carboxylic acid. So you'd have Cu2O. Right? So this would be a generic equation when it comes to the Benedict's test.
Benedict's Test Example 3
Video transcript
Here it says determine the product form when 3-ethylheptanal is treated with a basic copper(II) solution. So remember, a basic copper(II) solution would mean that we're undergoing the Benedict's test. And under the Benedict's test, we're just going to oxidize our aldehyde group into a carboxylic acid. So this structure, for the most part, would stay the same. We're just oxidizing that aldehyde group into a carboxylic acid group. So this represents our product from the oxidation of our aldehyde through the Benedict's test.
Determine the product formed when the following compound undergoes the Benedict's test.
No Reaction
Under the following test, which structure represents the product formed?
No Reaction
What was the starting material that created the following carboxylic acid product?
Draw the product when 2,3,5-trimethyloctanal is submerged in a basic copper (II) solution.
Do you want more practice?
Here’s what students ask on this topic:
What is the Tollens' test and how does it work?
The Tollens' test, also known as the silver mirror test, is used to detect the presence of aldehydes in a basic solution. It involves the reaction of an aldehyde with silver oxide (Ag2O) and aqueous ammonia (NH3). When heated, the aldehyde is oxidized to a carboxylic acid, and the silver ions are reduced to metallic silver, forming a silver precipitate. This precipitate deposits on the test tube, creating a reflective silver mirror. The reaction can be represented as:
 Created using AI
Created using AIWhat is the Benedict's test and how does it work?
The Benedict's test is used to detect the presence of aldehydes in a basic solution. It involves the reaction of an aldehyde with copper(II) ions (Cu2+) in a basic solution. The aldehyde is oxidized to a carboxylic acid, and the copper(II) ions are reduced to copper(I) oxide (Cu2O), which forms a brick-red precipitate. This color change indicates a positive Benedict's test. The reaction can be represented as:
 Created using AI
Created using AIWhat are the visual indicators of a positive Tollens' test?
A positive Tollens' test is indicated by the formation of a silver mirror on the inner surface of the test tube. This occurs because the aldehyde in the solution is oxidized to a carboxylic acid, and the silver ions (Ag+) are reduced to metallic silver (Ag), which deposits as a reflective layer. The reaction can be summarized as:
 Created using AI
Created using AIWhat are the visual indicators of a positive Benedict's test?
A positive Benedict's test is indicated by the formation of a brick-red precipitate in the solution. This occurs because the aldehyde in the solution is oxidized to a carboxylic acid, and the copper(II) ions (Cu2+) are reduced to copper(I) oxide (Cu2O), which precipitates out as a solid. The reaction can be summarized as:
 Created using AI
Created using AIHow do Tollens' and Benedict's tests differ in their chemical reactions?
Both Tollens' and Benedict's tests are used to detect aldehydes, but they differ in their chemical reactions and visual indicators. Tollens' test uses silver oxide (Ag2O) and aqueous ammonia (NH3) to oxidize aldehydes to carboxylic acids, producing a silver mirror as a visual indicator. The reaction is:
Benedict's test uses copper(II) ions (Cu2+) in a basic solution to oxidize aldehydes to carboxylic acids, producing a brick-red precipitate of copper(I) oxide (Cu2O). The reaction is:
 Created using AI
Created using AIYour GOB Chemistry tutor
- Which of the following will give a positive Tollens' test? (12.4) a. propanalb. ethanolc. ethyl methyl ether
- Which of the following will give a positive Tollens' test? (12.4) a. 1-propanolb. 2-propanolc. hexanal
- A fundamental difference between aldehydes and ketones is that one can be oxidized to carboxylic acids but the...
- Which of the following compounds will react with Tollens' reagent? With Benedict's reagent?a. Cyclopentanonb. ...
- Mention at least two simple chemical tests by which you can distinguish between benzaldehyde and benzoic acid.