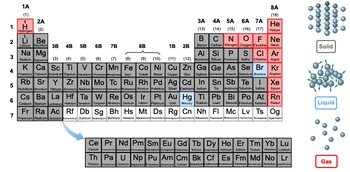
Hey everyone. So as we explore the periodic table and its elements, realize that at standard room temperature, which is approximately 25 degrees Celsius, and standard pressure, which is 1 atmosphere, the elements can exist under 3 states of matter. So we have our solids, our liquids, and our gases. Now if we take a look at these 3 states of matter a little bit more closely, we can talk about how their molecules orient themselves. For solids, they're kind of locked or frozen in place, and they're in direct contact with one another and because of that, they don't really conform to their container. As a result of this, we say that solids maintain a fixed shape and volume. Okay. So they're kind of frozen in place the way they are. Next, we have liquids. For liquids, the molecules are in close proximity to each other but they're able to move around more freely. So they're moving around one another, and because of this orientation, we can say that liquids can conform to the shape of the container, but not necessarily the volume of the container. So think about it like this—let's say we have a container that is 25 mL large, but we only have 10 mL of liquid. The liquid itself can conform to the shape of the container, but, try as it might, because there's not enough liquid, it can't fill up the entire volume of the container. So this is what happens with liquids. Gases, on the other hand, because their molecules are so far apart from each other, this opens up new properties or expanded properties when compared to solids and liquids. We won't talk about them here, but just realize because the molecules are further apart within gaseous states of matter, we can say that because of this, they assume both the shape and volume of the container.
Now if we take a look at the periodic table, we can see that a vast majority of the elements themselves are in solid form, like lithium, zinc, or sulfur. Some of them, very few of them, are in the form of gases. So, we can see that with hydrogen, nitrogen, oxygen, and chlorine; and the last batch is easy to remember because they're part of group 8A, which are our noble gases. So it's in the name; they're gases. And then finally, we can see that the rarest form is our liquids. Under standard room temperature and pressure, there are only 2 elements that exist in liquid form, and that will be mercury and bromine. Now you might also see that some elements are not color-coded as solids, liquids, or gases. We find those in the 7th row from Rf to Og. These elements are synthetically made within laboratories, and because they have such high masses, they're very unstable. So even at standard temperature and pressure, it's hard for us to determine what state they exist in. So they're very unpredictable. So for that reason, we don't give them any state of matter at 25 degrees Celsius or 1 atmosphere. So just remember that based on these different types of temperatures and pressures, a certain state of matter is preferred by different elements.