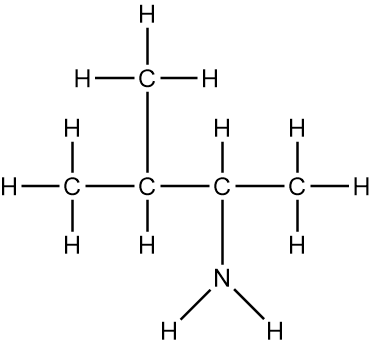
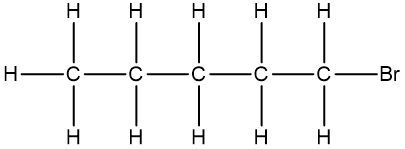
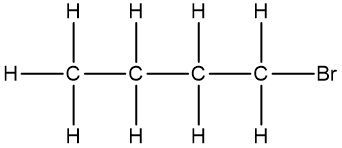
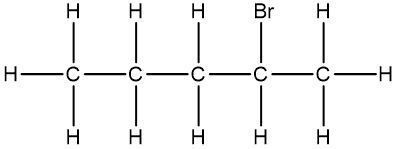
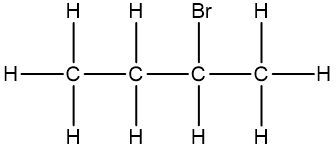
Now, the Condensed Formula shows how the atoms in a compound are bonded without showing all the bonds. Carbon and hydrogen atoms are grouped together in blocks. For example, in CH3, the 3 hydrogens on the compound are packed together, grouped together. In CH2, the 2 hydrogens on the carbon are grouped together, and in CH, the carbon and the hydrogen are grouped together.
If we take a look here, we have our structural formula. This formula shows all the bonds being created between our atoms. We're showing all the carbon-carbon bonds, and all the carbon-hydrogen bonds. Now, with condensed, we would package in this CH3, bringing those 3 hydrogens together, and the reason we draw it backward like this is to show that it's the carbons that are connecting to each other, and not hydrogen to carbon. Because if we wrote it the other way, it would almost look like the 3 hydrogens are connecting to my carbon, but they're not. It's the carbons that are connected to each other.
Here we have our CH2, so bring in those 2 hydrogens with the carbon, giving us CH2 here. And then here on the right end, we have CH3 again, bringing in those 3 hydrogens to give us CH3.
We can go even further and condense it even more by erasing the bonds that show the carbon-carbon linkages. So here, I erase this bond and this bond, and just bring everything in closer, so my fully condensed formula would be CH3CH2CH3. This is how we go from our structural formula to our condensed formula.