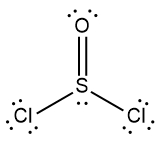
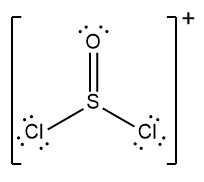
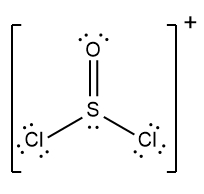
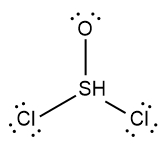
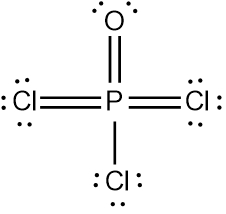
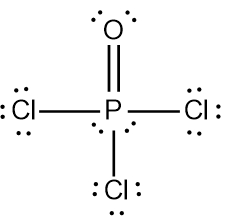
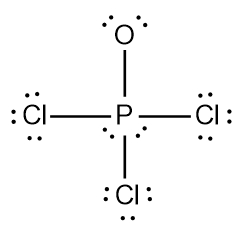
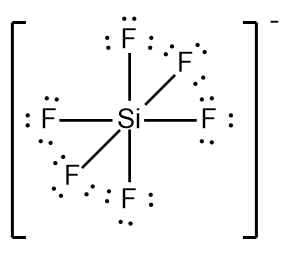
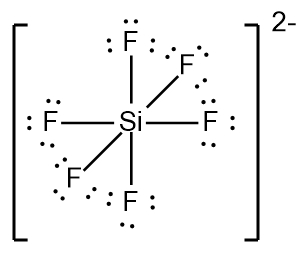
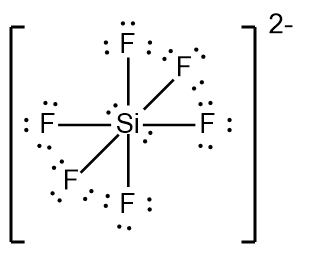
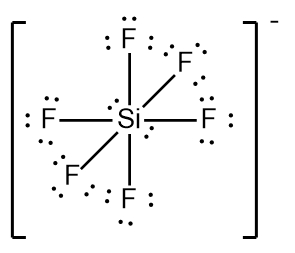
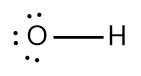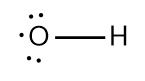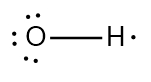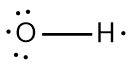In chemistry, the octet rule is a fundamental principle that states atoms tend to bond in such a way that they have eight electrons in their valence shell, achieving a stable electron configuration. However, certain elements can deviate from this rule and still maintain stability, particularly those in groups 2A through 6A of the periodic table.
For elements with incomplete octets, the number of electrons they can accommodate is determined by the formula:
Non-octet electrons = 2 × Group number
For example, elements in group 2A can have 4 electrons (2 × 2), while those in group 3A can have 6 electrons (2 × 3). This pattern continues, allowing group 5A elements to stabilize with 10 electrons, group 6A with 12, and so forth. Thus, elements can possess more than 8 electrons around them and still be stable, as seen in their Lewis dot structures.
Understanding these exceptions to the octet rule is crucial for predicting the behavior of certain elements during chemical bonding. It highlights the diversity of electron configurations and the complexity of molecular stability beyond the traditional octet framework.