In this video, we're going to talk about redox reactions, first from a General Chemistry perspective and then from an organic chemistry perspective. Recall that redox reactions, also known as oxidation-reduction reactions, involve the transference of an electron or electrons between reactants. This is our general chemistry definition of a redox reaction. In organic chemistry, it's a little bit different. Here, redox reactions involve increasing or decreasing the oxygen or hydrogen amount on a molecule. When we talk about oxidation, oxidation involves increasing the number of carbon-oxygen bonds on the molecule. If we're talking about reduction, that involves increasing the number of carbon-hydrogen bonds on the molecule.
If we take a look here at oxidation versus reduction, we're going to see that on the far left side, we have a hydrocarbon, in the form of methane, and then on the far right side, we have carbon dioxide. Notice that they're both grayed out. That's because we don't really deal with these two types of compounds when we're talking about oxidation versus reduction. We're going to focus on the middle portion, where we're going from an alcohol to what looks like an aldehyde to a carboxylic acid. That's the portion that we care about in terms of organic chemistry.
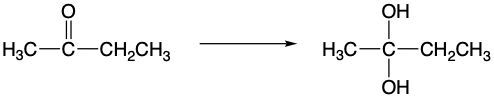
Now here, we can oxidize a hydrocarbon to our alcohol, and notice that we had no carbon-oxygen bonds initially, but our first oxidation step creates our first carbon-oxygen bond, applicable for alcohols. If we continue with our oxidation process, we started with one carbon-oxygen bond, and now we have two because we're double-bonded to that oxygen. This section here stands for aldehydes and ketones. If we continue oxidizing, we get to a carboxylic acid, and we go from having two carbon-oxygen bonds to now three. If we continued further, we would get carbon dioxide, which has four carbon-oxygen bonds. However, we do not concern ourselves with that portion because it does not represent an organic molecule for us.
Going the opposite way with reduction, we start out with the carboxylic acid, then move over to the aldehyde or ketone section. Here we notice that we go from three carbon-oxygen bonds to just two. Not just that, but we're increasing the number of carbon-hydrogen bonds. Initially, we had only one carbon-hydrogen bond, and now we're back to two. Continuing onward, instead of having two carbon-oxygen bonds, we have three, and we're at the alcohol phase. If we continued, we would have four carbon-hydrogen bonds. Again, we're not concerned with the extremes in this image; we don't concern ourselves with hydrocarbons or carbon dioxide. We operate in the highlighted section in the middle, between alcohols, aldehydes and ketones, and carboxylic acids.