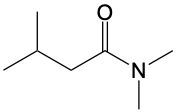
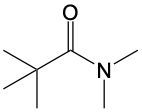
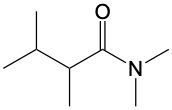
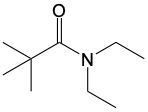
In this video, we're going to talk about amide formation. Now, under this type of reaction, a carboxylic acid and an amine undergo a condensation reaction to form an amide. Recall that a condensation reaction results in the loss of water. To do this, the carboxylic acid loses its OH group or OH portion, and the amine nitrogen loses its one hydrogen. So, for this to work, the nitrogen needs to possess at least one hydrogen. Here, for this to start, we need an H+ catalyst. This jump-starts the whole process.
If we take a look here at a typical amide formation general reaction, we have our carboxylic acid, and remember a squiggly line means it's connected to something else, which we don't care about at this moment. And then we have our amine, which again has a squiggly line, meaning it's connected to something else. Again, we're not concerned with that. We're focusing on the carboxylic acid portion and the amine portion. We're going to lose water; the OH from the carboxylic acid and the H from the amine, we lose those to produce water. This carbon needs to make up for the bond it just lost, and so does this nitrogen. So, what they do is bond to each other, and in that way, we create an amide. Remember, an amide is when we have a carbonyl connected, single bonded to a nitrogen group. This is how we form an amide through a condensation reaction between a carboxylic acid and an amine.