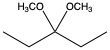
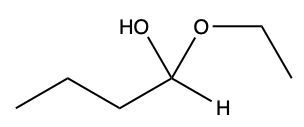
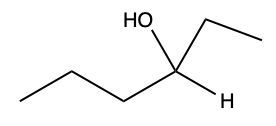
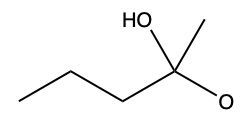
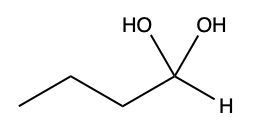
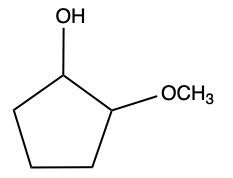
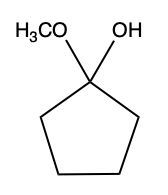
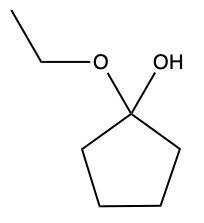
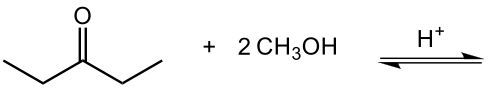Now here we can say that aldehydes and ketones react with alcohols to form hemiacetals and acetals. What exactly is a hemiacetal? Well, a hemiacetal is a compound with a hydroxy group, −OH, and an alkoxy group −OR on the same carbon atom. When we say R here, R is just a placeholder that represents carbon groups. This R could be a methyl group, an ethyl group, a ring, a more complex ring, or a long chain. It's just a placeholder for a carbon group. If we take a look here, we're starting out with this aldehyde. It reacts with this alcohol, and what we create is a carbon that has both an OH group and an OR group attached to it. So, we've just created a hemiacetal or hemiacetal, depending on how you want to pronounce it.
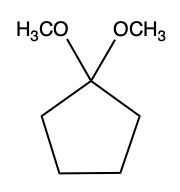
Now, what is a full acetal? Well, that's just a compound that possesses two alkoxy groups on the same carbon. This hemiacetal that we've created could react with another mole of an alcohol, causing the replacement of the OH group with an OR group. So now, you have a carbon connected to two OR groups. So what you have here is an acetal as your final compound. So just remember, aldehydes and ketones can both react with alcohols. If we react with one mole of alcohol, we create a hemiacetal. That hemiacetal can then react with a second mole of an alcohol to give us a full acetal compound.