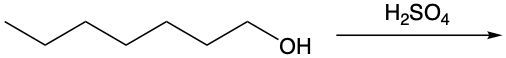
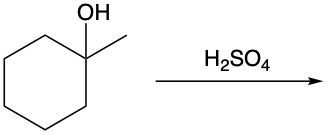
In this video, we're going to take a look at alcohol reactions, and in particular, dehydration reactions. Now, under dehydration reaction, this type of reaction involves sulfuric acid, which is H2SO4, reacting with an alcohol to form an alkene through the loss of water. To form the double bond, the alcohol carbon loses its OH, and its neighboring carbon loses an H atom.
If we take a look here at this example question or example reaction, we have our alcohol here; we're introducing our sulfuric acid. Here goes my alcohol carbon with its OH group, and we have this neighboring carbon and this neighboring carbon. Both of which happen to be methyl groups; they're both the same, so we can lose an H from either side. I decide to show it losing from the left side, but it could also happen on the right side since they're the same. So here, we're going to lose water, the water we lose is over here, and remember, carbon must continue to make 4 bonds. They each have lost a bond, one to hydrogen and one to OH. They need to replace that bond they've lost, so their only option is to make a double bond to one another. That's why we make an alkene as our product.
Remember, in a dehydration reaction, we're just introducing sulfuric acid to our alcohol in order to lose water and form an alkene.