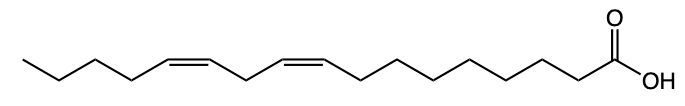
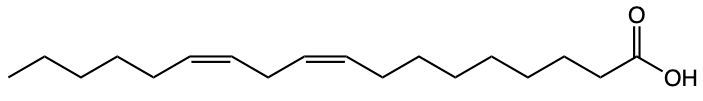
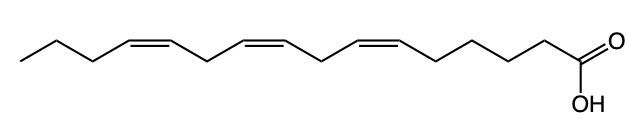
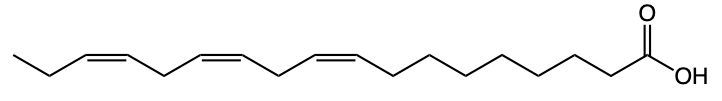
Fatty acids are essential components of lipids and typically consist of an even number of carbon atoms, ranging from 12 to 26. They can be represented by the general formula CnH2nCOOH, where n indicates the number of carbon atoms in the chain. These molecules are classified as amphipathic, meaning they possess both hydrophobic (non-polar) and hydrophilic (polar) regions.
The structure of a fatty acid includes a long hydrocarbon tail, which is hydrophobic due to its composition of only carbon and hydrogen atoms. This non-polar tail repels water, making it insoluble in aqueous environments. In contrast, the carboxylic acid group at one end of the fatty acid serves as the hydrophilic head, which is polar and thus interacts favorably with water molecules.
For example, Lauric acid, a common fatty acid, exemplifies this structure. Its long hydrocarbon tail is hydrophobic, while the carboxylic acid head is hydrophilic. The predominance of non-polar characteristics in fatty acids increases with the length of the hydrocarbon tail; therefore, longer fatty acids tend to be more non-polar overall. In summary, fatty acids consist of a hydrophobic hydrocarbon tail and a hydrophilic carboxylic acid head, highlighting their unique amphipathic nature.

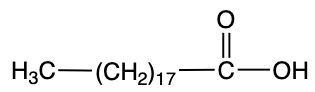 Storic acid
Storic acid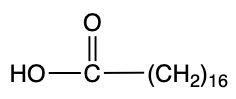 Palmitic acid
Palmitic acid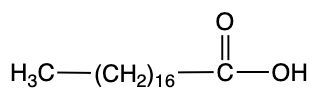 Stearic acid
Stearic acid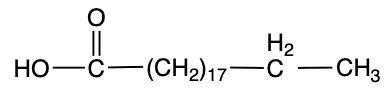 Palmitoleic acid
Palmitoleic acid