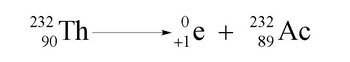Hey guys, in this new video, we're going to take a look at positron emission. So, here we're going to say positron emission occurs when an unstable nucleus emits a positron. Now, what's a positron? A positron is an anti-particle of the electron. Remember, the electron is represented by this. A positron is the opposite of that. So, it looks like an electron, but instead of it having a negative sign, it will have the opposite sign. So, it will be a positive electron. So, a positron is considered just a positive electron. I know this is weird, but again remember, we're dealing with nuclear reactions. So, a lot of unaccustomed things that we are not used to seeing do occur. And one of them is this positron emission. So, we're going to say here, here's our positron. Now, because we're talking about the word emission again, emission would mean decay, which means that this positron would be a product. So, let's think of an example. Here, Einstein has its own element named after him, Einsteinium. So, Einsteinium will deal with isotope 253 of einsteinium. So einsteinium is Es on our periodic table. It has an atomic number of 99. Here is the equation for the positron emission we discussed:
Es 253 99 → Es 253 98 + e +This would be an example of a positron decay or positron emission.