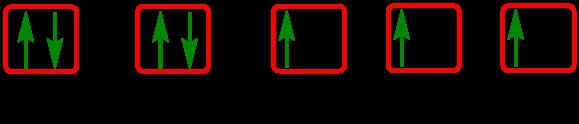
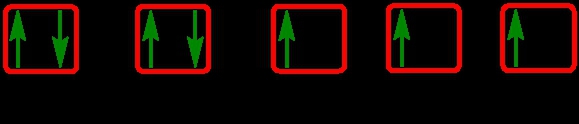
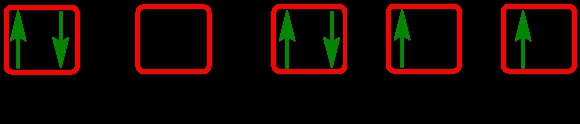
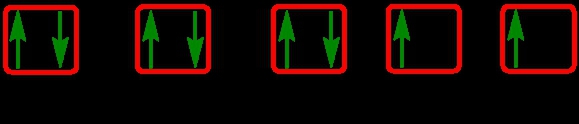
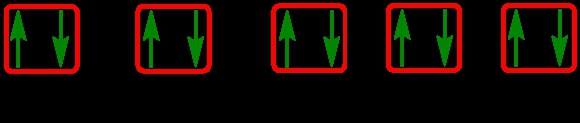
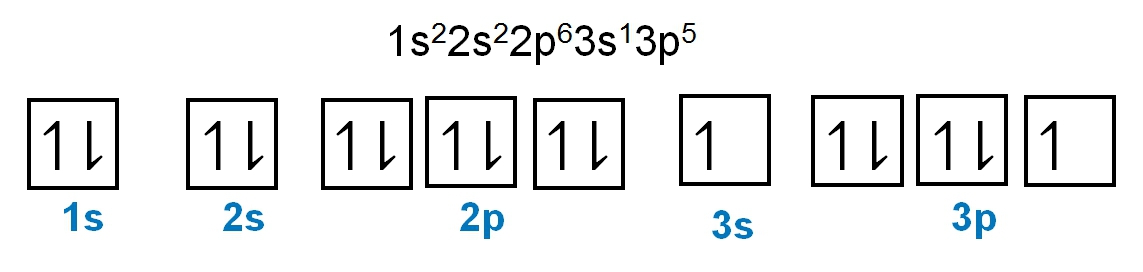
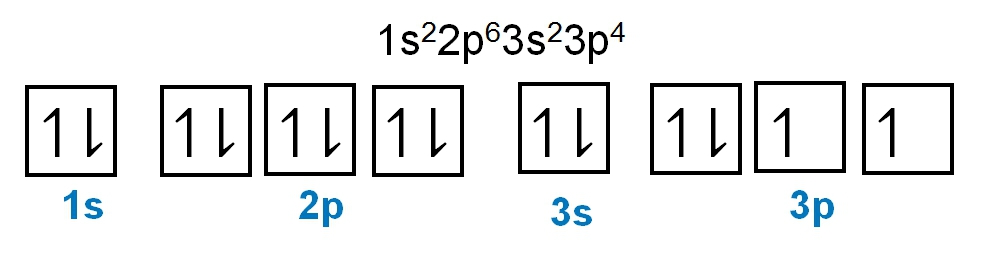
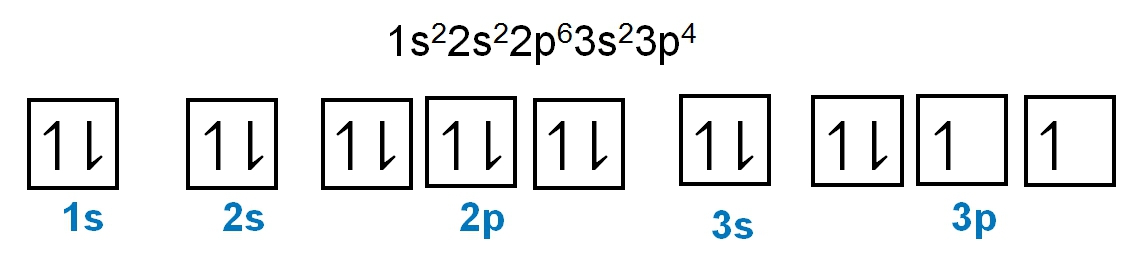
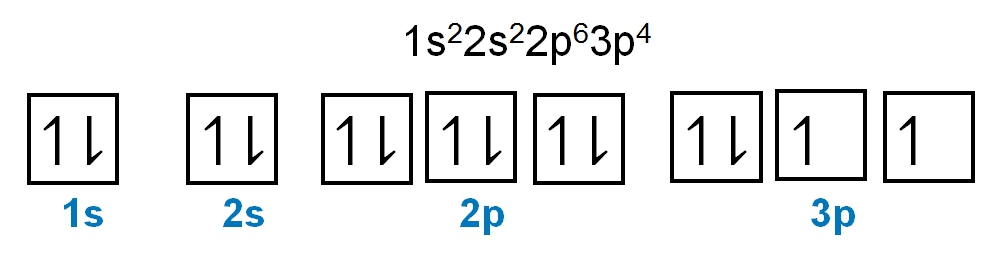
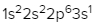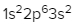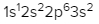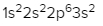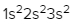As we stated earlier, the periodic law influences the electron arrangements of the elements, and the electron orbital diagrams are the visual representation within orbitals. Now we're going to say here we have what are called degenerate orbitals. These are electrons in the same set of orbitals having the same energy, and they're filled using Hund's rule. Now Hund's rule says that these degenerate orbitals are first half-filled before being totally filled. So if we take a look here, we have our s sublevel or s subshell. S can hold a maximum of 2 electrons. It has 1 orbital. Within that orbital, we have 2 electrons, one spins up, one spins down. So that would mean that the s sublevel has a maximum of 2 electrons.
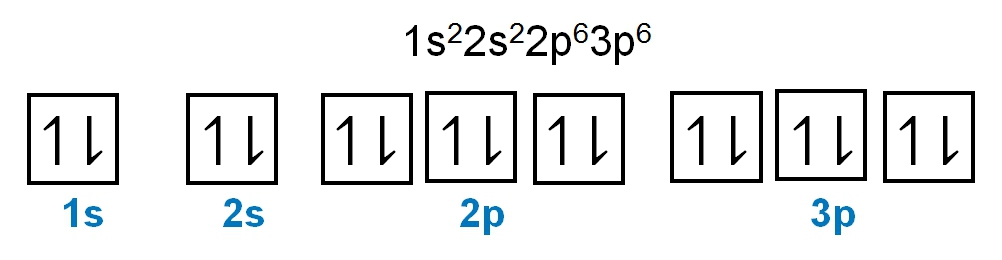
For the p sublevel, we have 3 orbitals. Following Hund's rule, we would half-fill them first. So we go up, up, up, and each orbital we know can hold a maximum of 2 electrons, so we come back around, down, down, down. So the p sublevel holds a maximum of 6 electrons. For the d sublevel, we have 5 orbitals here. Hund's rule says we half-fill them first since they're all d set of orbitals, they all have similar energy. So then we come back around, down, down, down, down, down for a total of 10 electrons. And finally, the f sublevel has 7 of these orbitals, half-fill them again according to Hund's rule. So when we half-fill them according to Hund's rule, come back around to totally fill them in. When we do that we get a total of 14 electrons.
So just remember the periodic law influences the electron arrangement of elements, and it's these orbital diagrams that depict the visual representation of electrons within any given orbital, based on subshell level or subshell letter. So just keep that in mind, s can hold a maximum of 2 electrons, p can hold up to 6, d can hold up to 10, and f can hold up to 14.